Monsalve et al.: Allergy
to Mustard Seeds - Importance of 2S Albumins as Food Allergens
Internet Symposium on Food Allergens
3(2):57-69 (2001) [http://www.food-allergens.de]
INTRODUCTION
| Type I allergies are common clinical disorders in developed countries,
food being one of the most relevant biological sources of these atopic
diseases. Many seed spices have been shown to be allergenic, and mustard
is one of the most significant in terms of its widespread use and allergenic
potency. The prevalence of food allergy varies with the dietary practice
of different populations and in countries such as France, where mustard
is extensively consumed, hypersensitivity to mustard seeds is increasing
and has been included among the most important food allergies (Andre et
al. 1994, Rance et al. 1999, Rance et al. 2000). In addition, several authors
have reported on its allergenic potency and this is of special relevance
if we consider that mustard is frequently present as an additive to many
meals or as hidden allergens in commercial foods (Panconesi et al. 1980,
Niinimaki & Hannuksela 1981, Monreal et al. 1992, Kanny et al. 1995,
Rance et al. 2000). Table 1 summarizes foods in
which mustard can be found. Although no reports on fatal anaphylactic reactions
to mustard seeds have been described, its allergenic potency must be seriously
taken into account. In fact, allergens from other seeds and nuts, such
as peanuts, walnuts or Brazil nuts, have been described as responsible
for causing fatal or near-fatal reactions in hypersensitive patients (Sampson
et al. 1992). Interestingly, these and other nuts contain allergens that
have been related to those of mustard seeds (Teuber et al. 1998). |
Table 1: List of foods in which mustard can
be found
| Babies and toddlers commercial foods |
| Barbecue sauce |
| Curry sauce |
| Cumberland sauce |
| Flours for flavouring fried fish or meat |
| Ketchup / Tomato sauce |
| Lubricant |
| Marinades |
| Mayonnaise |
| Meat (processed) |
| Meat (sausages) |
| Mustard powder (additive to foods) |
| Mustard sauce (commercial) |
| Mustard paste (home-made, combining the powder with wine
or vinegar and oil) |
| Piccalilli / Mustard pickle |
| Salad dressing |
| Salad oil |
| Spices for flavouring |
| Vinagrettes |
The information included in this table is taken from the
clinical cases reviewed in this paper (Table 2)
and from the Food Additive & Preservatives Allergy & Intolerance
Database (FAP AID) designed by Dr. Harris Steinman (http://zingsolutions.com/food/index.html#info) |
The major allergens of mustard seeds are storage proteins
of the 2S albumin class, which are abundant proteins of these seeds. They
are small (12 to 15 kDa) and basic proteins, generally composed of two
different polypeptide chains (of about 3-5 and 8-10 kDa) linked by disulphide
bridges. Allergenic 2S albumins have been isolated and characterized from
two species of mustard: yellow or white mustard (Sinapis alba L.)
(Menéndez-Arias et al. 1987, Menéndez-Arias et al. 1988)
and oriental mustard (Brassica juncea L.) (González de la
Peña et al. 1991). These two allergens have been named Sin a 1 and
Bra j 1, respectively, according to the nomenclature recommended by the
World Health Organization and the International Union of Immunological
Societies (King et al. 1994). Apart from these two species, black mustard
(Brassica nigra L.) is also frequently used as condiment (Meding
1985, Monreal et al. 1992). Table mustard is made from varying mixtures
of crushed seeds from different species according to the country of origin
or the manufacturer. For example, yellow and black mustard are the commonest
used in Europe, while the oriental mustard flour is most abundant in mustard
extracts from the USA or Japan (González de la Peña et al.
1991).
S. alba, B. juncea and B. nigra belong
to the Brassicaceae family, which also includes others such as Brassica
napus (rapeseed), Raphanus sativus (radish) and Arabidopsis
thaliana (thale cress). The sequences of many 2S albumins from these
species have been determined and studies on their gene expression regulation
and post-translational processing have been carried out (Crouch et al.
1983, Ericson et al. 1986, Krebbers et al. 1988, Monsalve & Rodríguez
1990, Raynal et al. 1991). Many reports refer to these storage proteins
as "napins" or napin-like proteins, since B. napus is the species
most extensively studied. One of its members, napin BnIb, has been isolated
and structurally characterized by our group, obtaining the first data on
the three-dimensional organization of a 2S albumin (Monsalve et al. 1991,
Rico et al. 1996). Besides the interest in these proteins for their storage
function and their allergenicity in mustard seeds, 2S albumins have also
been studied because of their antifungal activity (Terras et al. 1993,
Byczynska & Barciszewski 1999) and also because of their nutritional
qualities, since some of them have an unusually high content of essential
amino acids. As an example, the 2S albumins of the Brazil nut (Bertholletia
excelsa, family Lecythidaceae) are enriched in methionine (Gander
et al. 1991) and have been used to improve the nutritional quality of other
seeds (Guerche et al. 1990, Saalbach et al. 1994, Nordlee et al. 1996).
Nevertheless, in the case of transgenic soybean, the seeds obtained exhibited
allergenic properties due to the presence of 2S albumins expressed
by transferred DNA from Brazil nuts (Nordlee et al. 1996). As a matter
of fact, 2S albumins from different species have been described as allergens,
as will be shown later in this review.
Many reports on mustard hypersensitivity can be found
in the literature, but there has not been a review on mustard allergens.
In this manuscript we intend to survey the most relevant clinical studies
on this food allergy, as well as reviewing the biochemical and immunological
studies accumulated to date on the major mustard allergens. The structural
relationship of the major mustard allergens to other allergenic proteins
is presented and discussed in terms of possible common features that could
be related to their allergenic character.
CLINICAL DATA ON MUSTARD
SEED ALLERGY
Mustard allergy has been reported for years. Most of these
studies have analysed clinical cases of a very limited number of patients
with hypersensitivity symptoms after having ingested foods containing table
mustard or after inhaling or coming into contact with this spice as a powder.
Table
2 summarizes these studies and shows that most of them use skin prick
testing (SPT) and/or radioallergosorbent test (RAST) to confirm the allergenicity
to mustard seeds. Few studies have used histamine release assays and only
recently have oral challenges been carried out.
In spite of the relatively low number of individuals affected,
many authors have emphasized the severity of symptoms caused by mustard
seed allergens (Stricker et al. 1986, Monreal et al. 1992, Andre et al.
1994, Jorro et al. 1995), which induce strong anaphylactic reactions that
need urgent clinical intervention. This fact is of importance considering
that mustard is present in many foods (Table 1)
and that very often mustard products are hidden in foods (Panconesi et
al. 1980, Andre et al. 1994, Kanny et al. 1995, Rance et al. 2000). The
severity of symptoms caused by mustard has led some authors to suggest
not using oral challenges for testing this type of food allergy (Stricker
et al. 1986, Jorro et al. 1995). They state that SPT and RAST are sufficient
to demonstrate allergenicity, and systemic reactions can be avoided in
this way.
The most complete clinical studies on mustard allergy
have been those of Rance and colleagues (Rance & Dutau, 1997, Rance
et al. 1999, Rance et al. 2000). They tested many food allergens in a selected
population of 722 French children and adolescents (aged from one month
to 15 years) that were allergic to foods. Their work represents the first
analysis in which oral challenge is used to demonstrate mustard allergy.
Rance and Dutau (1997) use a labial food challenge (LFC) test as a reliable
technique to confirm the allergenicity of food in individuals suspected
to be hypersensitive from their case history, positive SPT and specific
serum IgE assays. It is a simple and rapid method to perform, and it is
associated with only low risks of systemic reactions. Positive results
clearly indicate the existence of food allergy, but the sensitivity of
this method is its major drawback: about 23% of mustard allergic individuals
had to be confirmed by single-blind placebo-controlled food challenges
(SBPCFC, Rance & Dutau, 1997). In Rance et al. 2000, the authors state
that the strong taste of mustard makes it very difficult to perform double-blind
placebo-controlled food challenges (DBPCFC), and that the use of lyophilized
capsules to circumvent this problem would prevent the first oropharyngeal
contact. Rance and colleagues mention that mustard allergy is increasing
in France, and that it affects to more than 10% of food-allergic patients.
They also state that this allergy is among the five most important food
allergies. In fact, eggs, peanuts, mustard, cow's milk and cod, in this
order, are responsible for 78% of the cases of food hypersensitivity in
their studies. These studies have also shown that allergic reactions to
mustard start early in life, usually below three years of age. Sensitization
could take place in utero or through breast-feeding, and frequent
contact with mustard in infancy could be explained by the presence of mustard
in baby foods in glass pots for babies and commercial foods for toddlers
(Rance et al. 2000). Therefore they suggest that mustard should be systematically
included in screening tests for food allergies and that labelling of foods
must be improved, since mustard is often hidden in food.
Table 2: Chronological compilation
of case reports and clinical studies on mustard allergy
Data on clinical symptoms, number of allergic patients
(with respect to the population tested, when applicable) and clinical tests
carried out are included. Abbreviations used: SPT = skin prick testing;
RAST = Radioallergosorbent test (generally Phadebas Pharmacia); LFC = Labial
Food Challenge; SBPCFC = single-blind placebo-controlled food challenge;
REIA = reversed enzyme immunoassay.
| Reference |
Clinical Symptoms of patients |
No. patients |
SPT |
RAST |
Other comments |
| Panconesi et al. 1980 |
Acute giant urticaria with oedema
of the glottis |
1 |
Yes |
Yes |
Mustard present as contamination |
| Meding 1985 |
Vesicular hand eczema |
1 |
Yes |
-- |
|
| Widstrom & Johansson 1986 |
Recurrent cases of acute severe
generalized urticaria and angioneurotic oedema |
1 |
Yes |
Yes |
|
| Stricker et al. 1986 |
Severe anaphylactic symptoms |
3 |
Yes |
-- |
|
| Dannaker & White 1987 |
Contact dermatitis symptoms (vesicular
dermatitis) |
1 |
Yes |
-- |
Allergy ascribed to isothiocyanates.
Patch tests were used. |
| Kavli & Moseng 1987 |
Contact urticaria |
3 |
Yes |
-- |
Also patch tests were performed.
Patients with occupational dermatitis |
| Menéndez-Arias et al. 1988
and 1990 |
Bronchial asthma and allergic rhinitis.
Anaphylactic shock in two patients. |
18 out of 107 |
Yes |
Yes |
Also REIA and histamine release
assays |
| Niinimaki et al. 1989 |
Gastric pain, rhinitis, atopic
dermatitis, or birch pollen allergy (in the whole population) |
29 out of 50 |
Yes |
Yes (only in 4 cases) |
Population with hypersensitivity
to spices. Cross-reactivity to birch pollen allergy proposed. |
| Diamond et al. 1990 |
Contact dermatitis |
1 |
-- |
-- |
Patch testing with different parts
of the plant (not with the seeds). Reaction adscribed to oil of mustard |
| Domínguez et al. 1990 |
Angioedema, urticaria or anaphylactic
shock |
7 |
Yes |
Yes |
Also REIA and histamine release
assays |
| Kohl & Frosch 1990 |
Irritant contact dermatitis |
1 |
Yes |
-- |
Temporary sensitization. Negative
SPT. |
| Vidal et al. 1991 |
Acute generalized urticaria, facial
and throat swelling, chest tightness |
2 |
-- |
Yes |
|
| Monreal et al. 1992 |
Anaphylactic reaction symptoms |
2 |
Yes |
Yes |
|
| Malet et al. 1993 |
Anaphylactic reaction symptoms |
2 |
Yes |
Yes |
Also histamine release and nasal
provocation tests |
| Andre et al. 1994 |
Anaphylactic reaction symptoms |
2 out of 60 |
Yes |
Yes |
|
| Niinimaki et al. 1995 |
Atopic dermatitis with respiratory
symptoms; respiratory symptoms; atopic dermatitis only; chronic hand eczema |
22 out of 49 |
Yes |
Yes |
Patient population with strong
SPT to spices |
| Kanny et al. 1995 |
Oral pruritus, oedema and generalized
urticaria |
1 |
Yes |
Yes |
|
| Jorro et al. 1995 |
Anaphylactic reaction symptoms |
3 |
Yes |
Yes |
|
| Monsalve et al. 1997 |
Wheezing with shortness of breath.
Oedema and pruritus in the lips, oral mucosa and the pharynx, and facial
urticaria |
1 |
Yes |
-- |
Also direct and inhibition ELISA
and immunoblotting |
| Rance & Dutau 1997
Rance et al. 1999 |
Atopic dermatitis. Laryngeal oedema,
angioedema, asthma and anaphylaxis. Oral allergy syndrome |
23 out of 142
49 out of 544 |
Yes |
Yes |
LFC and SBPCFC. Population of children
and adolescents |
| Rance et al. 2000 |
Atopic dermatitis. Urticaria and/or
angioedema. Asthma. Laryngeal oedema with oral alllergy syndrome. |
15 out of 36 |
Yes |
Yes |
SBPCFC to diagnose allergy (the
36 children were positive by SPT and/or RAST) |
CHARACTERIZATION OF MUSTARD SEED ALLERGENS
AND RAPESEED HOMOLOGOUS PROTEINS
Sin a 1, the major allergen of yellow mustard (Sinapis
alba) seeds was identified and characterized as a storage protein of
the 2S albumin class by our group (Menéndez-Arias et al. 1987, Menéndez-Arias
et al. 1988). The proteins that can be found in mustard seeds are mainly
12S globulins and 2S albumins. They were fractionated by size exclusion
chromatography, and tested for their allergenicity using sera of mustard-sensitive
patients by reverse enzyme immunoassays (REIA), histamine release and RAST
inhibition assays. The 2S albumin fraction was found to be the most potent
allergen in the extract, while the 12S fraction had a much lower response
(Menéndez-Arias et al. 1988). Sin a 1 was isolated from the 2S albumin
fraction by ion exchange chromatography and was characterized structurally
and immunologically. A protein with very similar structural and immunological
characteristics was obtained from oriental mustard (Brassica juncea)
seeds and named Bra j 1 (González de la Peña et al. 1991).
Sin a 1 was immunologically mapped by competition and
complementation assays using ten specific monoclonal antibodies (Menéndez-Arias
et al. 1990). According to these studies, Sin a 1 contains two immunodominant
regions to which most of these monoclonal antibodies are directed. These
regions are also allergenically relevant, since these IgG antibodies inhibited
IgE binding to Sin a 1 to some extent. Most of the monoclonal antibodies
bound to conformational antigenic determinants, and this was also the case
for the IgE binding epitopes. Nevertheless, one of the monoclonal antibodies
(named 2B3 in Menéndez-Arias et al. 1990) recognized a continuous
epitope on the large chain of Sin a 1. More defined studies led us to the
conclusion that this specific region contained an important allergenic
determinant of the molecule (Monsalve et al. 1993). It was also observed
that the epitope recognized by 2B3 was specific to mustard allergens (Sin
a 1 and Bra j 1), since this monoclonal antibody did not recognize the
most abundant 2S albumin of rapeseed (Bra n 1, as it has recently been
named in accordance with the IUIS Allergen Nomenclature Subcommittee, King
et al. 1994; this protein corresponds to napin BnIII in Monsalve &
Rodríguez, 1990). The difference in recognition of 2B3 was explained
by the variation in the primary structures of Sin a 1, Bra j 1 and Bra
n 1 (Monsalve et al. 1993), particularly by a histidine residue which is
present only in the mustard allergens (it corresponds to position 118 of
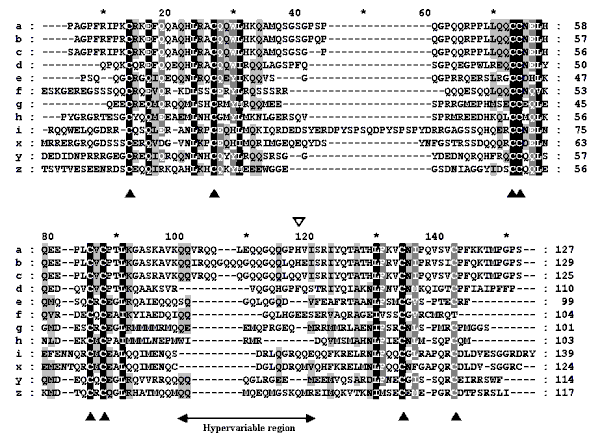
the aligned sequences of Figure 1, in which these
three proteins correspond to positions (a), (b) and (c), respectively).
The region to which this antibody binds is located in a segment that has
been named the "hypervariable region" of the 2S albumins (Krebbers et al.
1988, Monsalve et al. 1991, Raynal et al. 1991), because of the high variability
between the different members of this family in this stretch of the sequence.
Figure
1 clearly shows this variability: very long and variable gaps have
to be opened by the computer program, between positions 100 to 120 of the
aligned sequences, to achieve the best alignment (the other big gap which
appears in Figure 1 corresponds approximately to
the limits of the small and large chains that constitute most of these
2S albumins). Our group also emphasized the antigenic relevance of this
region after obtaining a three-dimensional model of Sin a 1, using the
atomic coordinates of napin BnIb as reference (Monsalve et al. 1995, Rico
et al. 1996). In both molecules, this segment corresponds to an exposed
loop which links two of the alpha-helices that make
up the scaffold of these molecules (Figure 2).
The sequence variability of this loop, which is evolutionarily allowed,
would mean that the folding process and the core structure are independent
of its length, as suggested by Krebbers et al. (1988), and it constitutes
an important antigenic region of the 2S albumins.
|
Figure 1: Multiple sequence alignment of allergenic 2S albumins
Letters on the left stand for the following allergens
(in correspondence with data in Table 3): (a)-Sin a 1; (b)-Bra j 1; (c)-Bra
n 1; (d)-BnIb; (e)-Ric c 1; (f)-Ric c 3; (g)-Ber e 1; (h)-SFA8; (i)-Ara
h 2; (x)-Ara h 6; (y)-Jug r 1; (z)-Mat5-D. Sequences of the mature proteins
(generally small and large chains together) were used for this alignment,
except for sequences (x), (y) and (z), for which only DNA data are known
(the deduced amino acid sequences were cut at the N-terminal ends to fit
the length of the longest mature protein). Filled triangles show the consensus
cysteine pattern of 2S albumins and the open triangle shows a specific
position mentioned in the text. Numbers on the right of the alignment correspond
to the sequence position of each molecule. ClustalW was the program used
(Thompson et al. 1994) and dashes represent gaps opened for the best alignment.
Shadowing of columns represents conservation among all the sequences: darker
backgrounds stand for the highest values of conservation. |
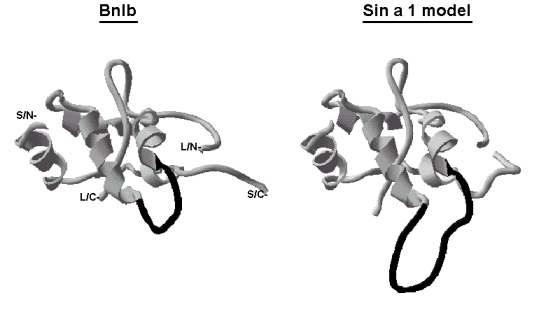
|
| Figure 2: Schematic ribbon representation of napin BnIb (PDB
code: 1PNB) and Sin a 1 model (Monsalve et al. 1995) The
loop that corresponds to the hypervariable region is drawn in black. Small
and large chains' termini of BnIb are labelled as S/N- and L/N- for the
NH2-terminal, respectively, and S/C- and L/C- for the COOH-terminal,
respectively. |
Other antigenic and allergenic determinants are yet to be studied in
detail, and to this end our group has obtained recombinant forms of Sin
a 1 (rSin a 1) or closely related counterparts of the 2S albumin class
(González de la Peña et al. 1993, González de la Peña
et al. 1996, Villalba et al. 2000). The importance of obtaining recombinant
products, apart from their being useful tools for the diagnosis and specific
immunotherapy of allergy or for studying structural features of proteins,
is evident in the case of mustard allergens, since 2S albumins are a very
complex protein fraction codified by multigenic families, each one therefore
represented by multiple isoforms. Recombinant production avoids manipulation
of microheterogeneous natural proteins. rSin a 1 obtained as a fusion protein
was shown to have most of the antigenic and allergenic determinants of
natural Sin a 1 (González de la Peña et al. 1996), although
the yield of soluble protein obtained was low. Recently, the cloning and
expression of recombinant BnIb (rBnIb) precursor was achieved in the yeast
Pichia
pastoris (Villalba et al. 2000). In this case, a high yield (50 mg
of protein per litre of culture) of a properly folded molecule was obtained.
The immunological and structural features of rBnIb were equivalent to those
of the natural protein. The recombinant molecule was bound by 50% of the
mustard-sensitive sera tested and therefore shares allergenic determinants
with mustard allergens. The availability of these recombinant molecules,
as well as of modified derivatives, will help to better define the IgE-epitopes
of mustard allergens and should also facilitate the design of hypoallergenic
variants which could be used in specific immunotherapy.
Our group has also identified and characterized the major allergen of
rapeseed (Brassica napus), Bra n 1 (Monsalve et al. 1997). This
protein is a close homologue of Sin a 1, with 94% of sequence similarity
after their alignment (Figure 1, Table
3). Bra n 1 has been defined as an occupational allergen that affected
a patient who worked in a feed processing plant, handling rapeseed flour,
cereals, soybean, and sunflower seeds, among other foods. The patient gradually
became hypersensitive to rapeseed flour, and the disease developed into
a strong food hypersensitivity to mustard-containing products. The antigenic
properties of Bra n 1 were studied, in comparison to those of Sin a 1,
by using sera from mustard- and rapeseed-sensitive patients. These studies
allowed us to conclude that these two proteins share common antigenic and
allergenic determinants. Several authors had previously reported on the
cross-reactivity between the proteins of rape and mustard seeds (Meding,
1985, Widstrom & Johansson, 1986, Monreal et al. 1992). Therefore,
homologous proteins of Sin a 1 and Bra j 1 are also potentially allergenic
for mustard-sensitive patients.
Table 3: Sequence and molecular
data of 2S albumins that have been defined as allergens
Sequence data of mature proteins are included whenever
possible, complemented by data on the precursors deduced from cDNAs when
these are complete. Accession numbers correspond to SwissProt and TrEMBL
databases, except for L77197, which corresponds to Genbank. The proteins
are listed in the same order as that of Figure 1,
with the same reference letter.
| Allergen Name |
Species and common
name |
Database Accession
Number |
Precursor Length
(*) |
Mature Chains'
Size (**) |
Mr of mature protein
(Da) |
Ref. (***) |
Ident.% (Simil.%) |
| Sin a 1 |
Sinapis alba (yellow mustard) |
P15322 |
|
39 / 88 |
14180 |
(a) |
(****) |
| Bra j 1 |
Brassica juncea (oriental
mustard) |
P80207 |
|
37 / 92 |
14644 |
(b) |
86 (91) |
| Bra n 1 |
Brassica napus (rapeseed) |
P80208 |
|
37 / 88 |
14035 |
(c) |
92 (94) |
| BnIb |
Brassica napus (rapeseed) |
P24565 |
|
31 / 79 |
12691 |
(d) |
47 (61) |
| Ric c 1 |
Ricinus communis (castor
bean) |
P01089 |
258 (*) |
34 / 65 |
11212 |
(e) |
25 (43) |
| Ric c 3 |
Ricinus communis (castor
bean) |
P01089 |
258 (*) |
37 / 70 |
12032 |
(f) |
20 (35) |
| Ber e 1 |
Bertholletia excelsa (brazil
nut) |
P04403 |
146 |
28 / 73 |
12218 |
(g) |
18 (39) |
| SFA8 |
Helianthus annuus (common
sunflower) |
P23110 |
141 |
103 |
12155 |
(h) |
14 (28) |
| Ara h 2 |
Arachis hypogaea (peanut) |
L77197 |
157 |
138 |
16637 |
(i) |
14 (31) |
| Ara h 6 |
Arachis hypogaea (peanut) |
Q9SQG5 |
129 |
|
|
(x) |
17 (34) |
| Jug r 1 |
Juglans regia (English walnut) |
P93198 |
139 |
|
|
(y) |
21 (38) |
| Mat5-D |
Gossypium hirsutum
(upland cotton) |
Q39787 |
139 |
(27 / 76) |
|
(z) |
12 (31) |
Footnotes:
(*) Size expressed as number of amino acids (only
shown when the complete precursor is known). Ric c 1 and Ric c 3 are codified
by the same precursor.
(**) Defined as the number of amino acids of the
small and large chains. In the case of SFA8 and Ara h 2, only one chain
constitutes the mature protein. For Mat5-D, the predicted size of chains
is shown.
(***) Letters used to identify the different allergenic
proteins correspond to the following bibliographic references: (a) - (Menéndez-Arias
et al., 1988); (b) - (Monsalve et al., 1993); (c) - (Monsalve et al., 1997);
(d) - (Monsalve et al., 1991, Villalba et al., 2000); (e) and (f) - (Irwin
et al., 1990, Bashir et al., 1998); (g) - (Ampe et al., 1986, Nordlee et
al., 1996); (h) - (Kelly & Hefle, 2000); (i) - (Stanley et al., 1997);
(x) - (Kleber-Janke et al., 1999); (y) - (Teuber et al., 1998); (z) - (Youle
& Huang, 1979, Galau et al., 1992).
(****) Identity (Ident.%) and similarity (Simil.%)
percentages correspond to the alignment shown in Figure
1 and are referred to the pairwise comparison with Sin a 1. Identities
correspond to exact matches and similarities to conservative changes.
STRUCTURAL FEATURES
AND ALLERGENICITY OF 2S ALBUMINS
In the last few years, 2S albumins from other species
have also been described as allergens, as is shown in Table
3. The presence of these allergenic proteins in seeds of numerous species
has led to the proposal that these storage proteins can be considered "universal
allergens" (Teuber et al. 1998). This is so even if they do not necessarily
cross-react and even though their primary structures differ considerably
in some cases. As is shown in Table 3 and Figure
1, the percentage of sequence identity of some of these 2S albumins
is as low as 14%. This is the case with SFA8, a sunflower 2S albumin, and
Ara h 2, a major allergen of peanut that has a prevalence of 90% in patients
allergic to peanut (Stanley et al. 1997). These two proteins are in the
borderline of being considered homologous to Sin a 1 (their percentages
of similarity reach 29 and 31%, respectively). Both SFA8 and Ara h 2 are
the only allergenic 2S albumins that have been described as being constituted
of a single polypeptide chain. In any case, heterodimeric 2S albumins are
synthesized as single precursors that are proteolytically cleaved during
maturation with the loss of an internal peptide, apart from the removal
of other N- and C-terminal regions. The alignment shown in Figure
1 has been carried out with the sequences of mature allergenic 2S albumins,
except for the sequences (x), (y) and (z), which correspond to the unprocessed
precursors of these proteins. Hence, the identity and similarity values
shown in Table 3 for these proteins are lower than
the ones that could be obtained with the mature proteins.
In spite of the differences mentioned, the evolutionary relationship
of 2S albumins has been pointed out by several authors (Raynal et al. 1991,
Bashir et al. 1998, Teuber et al. 1998). In fact, one constant feature
in all the storage proteins belonging to the 2S albumin class is the presence
of cysteine residues distributed according to a conserved pattern (...C...C...
/ ...CC...CxC...C...C...; see Figure 1). These
residues generally form four disulphide bridges that covalently link both
polypeptide chains, and are also present in the one-chain SFA8 (Egorov
et al. 1996). The existence of this cysteine pattern has also been described
in other storage proteins, such as the sulphur-rich prolamins and alpha-amylase
inhibitors from cereals (Kreis et al. 1985, Shewry et al. 1995). Therefore,
the existence of a common ancestor has been proposed not only for the 2S
albumins, but also for other groups of storage proteins belonging to groups
phylogenetically more distant.
The determination of the global fold of napin BnIb (Rico et al. 1996),
the only 2S albumin for which three-dimensional data are available, revealed
that these storage proteins are structurally similar to two different families
of proteins: the non specific Lipid Transfer Proteins (LTPs) and the cereal
alpha-amylase/trypsin inhibitors (Rico et al. 1996, Behnke et al. 1998).
As a matter of fact, these three groups of proteins constitute a super-family
of the "all alpha" protein class, according to the classification of the
SCOP structural database (Murzin et al. 1995). These three families are
defined as disulphide-rich, with a common fold of "four helices, folded
leaf, right-handed superhelix". There is no apparent relation among the
primary structures of these proteins, but if they are aligned considering
their three-dimensional organization, it is possible to match a cysteine
pattern that resembles the same distribution mentioned above. The cysteine
residues that constitute this pattern have also been described as being
involved in disulphide bridges in a similar distribution to that of the
2S albumins (Egorov et al. 1996, Rico et al. 1996, Poznanski et al. 1999).
These structural similarities have led different authors (Rico et al. 1996,
Behnke et al. 1998, Pandya et al. 2000) to suggest the existence of a common
ancestor in plants that must have diverged to proteins with different functional
activities but with a conserved common conformation. This proposal is of
utmost interest if we consider that, apart from the 2S albumins, many members
of each of these families have been defined as allergenic proteins. An
important case is that of the pan-allergenic LTPs, which are very potent
allergens present in different fruits (Asero et al. 2000). Belonging to
the family of the LTPs, the hydrophobic protein of soybean (HPS) from the
hull of Glycine max seeds has also been defined as an allergen (Baud
et al. 1993, González et al. 1995). On the other hand, many studies
have reported that the allergens that induce baker's asthma disease are
seed proteins belonging to the alpha-amylase/trypsin inhibitors family
(Barber et al. 1989, Gómez et al. 1990, Izumi et al. 1992, García-Casado
et al. 1995, Behnke et al. 1998).
The structural relationship between such a wide group of important plant
allergenic proteins opens the question of whether there are any structural
features that can be related to their allergenic character. Their compactness
due to the disulphide bridges packing would help to give to these proteins
a special resistance to proteolysis and thermal denaturation. This property
has been observed for 2S albumins (Domínguez et al. 1990, Oñaderra
et al. 1994, Astwood et al. 1996) and also for LTPs (Asero et al. 2000).
In the case of food allergens, this could allow these proteins to reach
gastrointestinal tract almost intact. In fact, digestibility has been emphasized
as a key factor for food allergens (Astwood et al. 1996, Aalberse, 2000).
Moreover, their allergenicity could be favoured by the interaction of these
proteins with membranes. LTPs clearly interact with membranes due to their
functionality (Wirtz, 1991, Poznanski et al. 1999). In the case of 2S albumins,
we have demonstrated that there is a strong interaction of Sin a 1 with
phospholipid vesicles (Oñaderra et al. 1994). In this work we proposed
that this interaction could underlie its allergenic character as a food
allergen. In fact, this could result in an increased cellular uptake, a
reduced neutralization by secretory antibodies and a decreased degradation
in the blood stream.
Finally, it is also worth mentioning that, to our knowledge, no cross-reactivity
between these groups of allergenic proteins has been described. Their common
global fold, which is kept compact by a similar network of disulphide bridges,
has regions of sequential variability located mainly in the loop regions
(Rico et al. 1996). This variability could be the reason for the lack of
cross-reactivity between them. This is in agreement with Rod Aalberse,
who states that proteins with a similar fold are not necessarily cross-reactive
(Aalberse, 2000).
The elucidation of these questions will require many more studies. Future
structural and immunological studies in this wide group of allergenic proteins
will certainly reveal which of these structural properties, if any, are
the most significant for inducing allergic reactions and could give some
clues to enhance understanding of the molecular basis of food allergy.
ACKNOWLEDGEMENTS
The work carried out by our group has been supported by
grants PB89/087, PB92/0195, PM95/0074 and PM98/0094 from the Dirección
General de Investigación Científica y Técnica (M.E.C.;
Spain).
REFERENCES
-
Aalberse R (2000) Structural biology of allergensJ
Allergy Clin Immunol 106:228-38
-
Ampe C, Van Damme J, de Castro LA, Sampaio MJ, Van Montagu
M, Vandekerckhove J (1986) The amino-acid sequence of the 2S sulphur-rich
proteins from seeds of Brazil nut (Bertholletia excelsa H.B.K.)Eur
J Biochem 159:597-604
-
Andre F, Andre C, Colin L, Cacaraci F, Cavagna S (1994) Role
of new allergens and of allergens consumption in the increased incidence
of food sensitizations in France Toxicology 93:77-83
-
Asero R, Mistrello G, Roncarolo D, de Vries SC, Gautier MF,
Ciurana CL, Verbeek E, Mohammadi T, Knul-Brettlova V, Akkerdaas JH, Bulder
I, Aalberse RC, van Ree R (2000) Lipid transfer protein: a pan-allergen
in plant-derived foods that is highly resistant to pepsin digestionInt
Arch Allergy Immunol 122:20-32
-
Astwood JD, Leach JN, Fuchs RL (1996) Stability of food
allergens to digestion in vitro Nat Biotechnol 14:1269-73
-
Barber D, Sánchez-Monge R, Gómez L, Carpizo
J, Armentia A, López-Otín C, Juan F, Salcedo G (1989) A
barley flour inhibitor of insect alpha-amylase is a major allergen associated
with baker's asthma disease FEBS Lett 248:119-22
-
Bashir ME, Hubatsch I, Leinenbach HP, Zeppezauer M, Panzani
RC, Hussein IH (1998) Ric c 1 and Ric c 3, the allergenic 2S albumin
storage proteins of Ricinus communis: complete primary structures
and phylogenetic relationships Int Arch Allergy Immunol 115:73-82
-
Baud F, Pebay-Peyroula E, Cohen-Addad C, Odani S, Lehmann
MS (1993) Crystal structure of hydrophobic protein from soybean; a member
of a new cysteine-rich family J Mol Biol 231:877-87
-
Behnke CA, Yee VC, Trong IL, Pedersen LC, Stenkamp RE, Kim
SS, Reeck GR, Teller DC (1998) Structural determinants of the bifunctional
corn Hageman factor inhibitor: x-ray crystal structure at 1.95 A resolutionBiochemistry37:15277-88
-
Byczynska A, Barciszewski J (1999) The biosynthesis, structure
and properties of napin - the storage protein from rape seeds J
Plant Physiol 154:417-25
-
Crouch ML, Tenbarge KM, Simon AE, Ferl R (1983) cDNA clones
for Brassica napus seed storage proteins: evidence from nucleotide
sequence analysis that both subunits of napin are cleaved from a precursor
polypeptide J Mol Appl Genet 2:273-83
-
Dannaker CJ, White IR (1987) Cutaneous allergy to mustard
in a salad maker Contact Dermatitis 16:212-4
-
Diamond SP, Wiener SG, Marks JG (1990) Allergic contact
dermatitis to nasturtium Dermatol Clin 8:77-80
-
Domínguez J, Cuevas M, Ureña V, Munoz T, Moneo
I (1990) Purification and characterization of an allergen of mustard
seed Ann Allergy 64:352-7
-
Egorov TA, Odintsova TI, Musolyamov A, Fido R, Tatham AS,
Shewry PR (1996) Disulphide structure of a sunflower seed albumin: conserved
and variant disulphide bonds in the cereal prolamin superfamily FEBS
Lett 396:285-8
-
Ericson ML, Rodin J, Lenman M, Glimelius K, Josefsson LG,
Rask L (1986) Structure of the rapeseed 1.7 S storage protein, napin,
and its precursor J Biol Chem 261:14576-81
-
Galau GA, Wang HY-C, Hughes DW (1992) Cotton Mat5-A (C164)
gene and Mat5-D cDNAs encoding methionine-rich 2S albumin storage proteinsPlant
Physiol 99:779-82
-
Gander ES, Holmstroem KO, De Paiva GR, De Castro LA, Carneiro
M, Grossi de Sa MF (1991) Isolation, characterization and expression
of a gene coding for a 2S albumin from Bertholletia excelsa (Brazil
nut) Plant Mol Biol 16:437-48
-
García-Casado G, Armentia A, Sánchez-Monge
R, Sánchez LM, López-Otín C, Salcedo G (1995) A
major baker's asthma allergen from rye flour is considerably more active
than its barley counterpart FEBS Lett 364:36-40
-
Gómez L, Martín E, Hernández D, Sanchez-Monge
R, Barber D, del Pozo V, de Andrés B, Armentia A, Lahoz C, Salcedo
G, al. e (1990) Members of the alpha-amylase inhibitors family from
wheat endosperm are major allergens associated with baker's asthmaFEBS
Lett 261:85-8
-
González de la Peña MA, Menéndez-Arias
L, Monsalve RI, Rodríguez R (1991) Isolation and characterization
of a major allergen from oriental mustard seeds, Bra j I Int Arch
Allergy Appl Immunol 96:263-70
-
González de la Peña MA, Villalba M, García-Lopez
JL, Rodríguez R (1993) Cloning and expression of the major allergen
from yellow mustard seeds, Sin a I Biochem Biophys Res Commun190:648-53
-
González de la Peña MA, Monsalve RI, Batanero
E, Villalba M, Rodríguez R (1996) Expression in Escherichia
coli of Sin a 1, the major allergen from mustard Eur J Biochem237:827-32
-
González R, Varela J, Carreira J, Polo F (1995) Soybean
hydrophobic protein and soybean hull allergy Lancet 346:48-9
-
Guerche P, De Almeida ER, Schwarztein MA, Gander E, Krebbers
E, Pelletier G (1990) Expression of the 2S albumin from Bertholletia
excelsa in Brassica napus Mol
Gen Genet 221:306-14
-
Irwin SD, Keen JN, Findlay JB, Lord JM (1990) The Ricinus
communis 2S albumin precursor: a single preproprotein may be processed
into two different heterodimeric storage proteins Mol Gen Genet222:400-8
-
Izumi H, Adachi T, Fujii N, Matsuda T, Nakamura R, Tanaka
K, Urisu A, Kurosawa Y (1992) Nucleotide sequence of a cDNA clone encoding
a major allergenic protein in rice seeds. Homology of the deduced amino
acid sequence with members of alpha-amylase/trypsin inhibitor familyFEBS
Lett 302:213-6
-
Jorro G, Morales C, Braso JV, Pelaez A (1995) Mustard
allergy: three cases of systemic reaction to ingestion of mustard sauceJ
Investig Allergol Clin Immunol 5:54-6
-
Kanny G, Fremont S, Talhouarne G, Nicolas JP, Moneret-Vautrin
DA (1995) Anaphylaxis to mustard as a masked allergen in "chicken dips"Ann
Allergy Asthma Immunol 75:340-2
-
Kavli G, Moseng D (1987) Contact urticaria from mustard
in fish-stick production Contact dermatitis 17:153-5
-
Kelly JD, Hefle SL (2000) 2S methionine-rich protein (SSA)
from sunflower seed is an IgE-binding protein Allergy 55: 556-9
-
King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills
TA, Thomas W (1994) Allergen nomenclature. WHO/IUIS Allergen Nomenclature
Subcommittee Int Arch Allergy Immunol 105:224-33
-
Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker
WM (1999) Selective cloning of peanut allergens, including profilin
and 2S albumins, by phage display technology Int Arch Allergy Immunol119:265-74
-
Kohl PK, Frosch PJ (1990) Irritant contact dermatitis
induced by a mustard compress Contact dermatitis 23:189-190
-
Krebbers E, Herdies L, De Clercq A, Seurinck J, Leemans J,
Van Damme J, Segura M, Gheysen G, Van Montagu MM, Vandekerckhove JS (1988)
Determination
of the processing sites of an Arabidopsis 2S albumin and characterization
of the complete gene family Plant Physiol87:859-66
-
Kreis M, Forde BG, Rahman S, Miflin BJ, Shewry PR (1985)
Molecular
evolution of the seed storage proteins of barley, rye and wheat J
Mol Biol 183:499-502
-
Malet A, Valero A, Lluch M, Bescos M, Amat P, Serra E (1993)
Hypersensitivity
to mustard seed Allergy 48:62-3
-
Meding B (1985) Immediate hypersensitivity to mustard
and rape Contact Dermatitis 13:121-2
-
Menéndez-Arias L, Monsalve RI, Gavilanes JG, Rodríguez
R (1987) Molecular and spectroscopic characterization of a low molecular
weight seed storage protein from yellow mustard (Sinapis alba L.)Int
J Biochem 19:899-907
-
Menéndez-Arias L, Moneo I, Domínguez J, Rodríguez
R (1988) Primary structure of the major allergen of yellow mustard (Sinapis
alba L.) seed, Sin a I Eur J Biochem 177:159-66
-
Menéndez-Arias L, Domínguez J, Moneo I, Rodríguez
R (1990) Epitope mapping of the major allergen from yellow mustard seeds,
Sin a I Mol Immunol 27:143-50
-
Monreal P, Botey J, Pena M, Marin A, Eseverri JL (1992) Mustard
allergy. Two anaphylactic reactions to ingestion of mustard sauce Ann
Allergy 69:317-20
-
Monsalve RI, Rodríguez R (1990) Purification and
characterization of proteins from the 2S fraction from seeds of the Brassicaceae
familyJ
Exp Botany 41:89-94
-
Monsalve RI, López-Otín C, Villalba M, Rodríguez
R (1991) A new distinct group of 2 S albumins from rapeseed. Amino acid
sequence of two low molecular weight napins FEBS Lett 295:207-10
-
Monsalve RI, González de la Peña MA, Menéndez-Arias
L, López-Otín C, Villalba M, Rodríguez R (1993) Characterization
of a new oriental-mustard (Brassica juncea) allergen, Bra j IE:
detection of an allergenic epitope Biochem J 293:625-32
-
Monsalve RI, Villalba M, Rico M, González de la Peña
M, Batanero E, Rodríguez R (1995) Three dimensional approach
to the structure of the yellow mustard allergen Allergy 50:136S
(abstract)
-
Monsalve RI, González de la Peña MA, López-Otín
C, Fiandor A, Fernández C, Villalba M, Rodríguez R (1997)
Detection,
isolation and complete amino acid sequence of an aeroallergenic protein
from rapeseed flour Clin Exp Allergy 27:833-41
-
Murzin AG, Brenner SE, Hubbard T, Chothia C (1995) SCOP:
a structural classification of proteins database for the investigation
of sequences and structures J Mol Biol 247:536-40
-
Niinimaki A, Bjorksten F, Puukka M, Tolonen K, Hannuksela
M (1989) Spice allergy: results of skin prick tests and RAST with spice
extracts Allergy 44:60-5
-
Niinimaki A, Hannuksela M (1981) Immediate skin test reactions
to spices Allergy 36:487-93
-
Niinimaki A, Hannuksela M, Makinen-Kiljunen S (1995) Skin
prick tests and in vitro immunoassays with native spices and spice extractsAnn
Allergy Asthma Immunol 75:280-6
-
Nordlee JA, Taylor SL, Townsend JA, Thomas LA, Bush RK (1996)
Identification
of a Brazil-nut allergen in transgenic soybeans N Engl J Med334:688-92
-
Oñaderra M, Monsalve RI, Mancheño JM, Villalba
M, Martínez del Pozo A, Gavilanes JG, Rodríguez R (1994)
Food
mustard allergen interaction with phospholipid vesicles Eur J Biochem225:609-15
-
Panconesi E, Sertoli A, Fabbri P, Giorgini S, Spallanzani
P (1980) Anaphylactic shock from mustard after ingestion of pizzaContact
Dermatitis 6:294-5
-
Pandya MJ, Sessions RB, Williams PB, Dempsey CE, Tatham AS,
Shewry PR, Clarke AR (2000) Structural characterization of a methionine-rich,
emulsifying protein from sunflower seed Proteins 38:341-9
-
Poznanski J, Sodano P, Suh SW, Lee JY, Ptak M, Vovelle F
(1999) Solution structure of a lipid transfer protein extracted from
rice seeds. Comparison with homologous proteins Eur J Biochem259:692-708
-
Rance F, Dutau G (1997) Labial food challenge in children
with food allergy Pediatr Allergy Immunol 8:41-4
-
Rance F, Kanny G, Dutau G, Moneret-Vautrin DA (1999) Food
hypersensitivity in children: clinical aspects and distribution of allergensPediatr
Allergy Immunol 10:33-8
-
Rance F, Abbal M, Dutau G (2000) Mustard allergy in childrenAllergy
55:496-500
-
Raynal M, Depigny D, Grellet F, Delseny M (1991) Characterization
and evolution of napin-encoding genes in radish and related crucifersGene99:77-86
-
Rico M, Bruix M, González C, Monsalve RI, Rodríguez
R (1996) 1H NMR assignment and global fold of napin BnIb,
a representative 2S albumin seed protein Biochemistry 35:15672-82
-
Saalbach I, Pickardt T, Machemehl F, Saalbach G, Schieder
O, Muntz K (1994) A chimeric gene encoding the methionine-rich 2S albumin
of the Brazil nut (Bertholletia excelsa H.B.K.) is stably expressed
and inherited in transgenic grain legumesMol Gen Genet 242:226-36
-
Sampson HA, Mendelson L, Rosen JP (1992) Fatal and near-fatal
anaphylactic reactions to food in children and adolescents N Engl
J Med 327:380-4
-
Shewry PR, Napier JA, Tatham AS (1995) Seed storage proteins:
structures and biosynthesis Plant Cell 7:945-56
-
Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell
G, Helm RM, West CM, Bannon GA (1997) Identification and mutational
analysis of the immunodominant IgE binding epitopes of the major peanut
allergen Ara h 2 Arch Biochem Biophys 342:244-53
-
Stricker WE, Anorve-Lopez E, Reed CE (1986) Food skin
testing in patients with idiopathic anaphylaxis J Allergy Clin Immunol77:516-9
-
Terras FR, Torrekens S, Van Leuven F, Osborn RW, Vanderleyden
J, Cammue BP, Broekaert WF (1993) A new family of basic cysteine-rich
plant antifungal proteins from Brassicaceae species FEBS
Lett 316:233-40
-
Teuber SS, Dandekar AM, Peterson WR, Sellers CL (1998) Cloning
and sequencing of a gene encoding a 2S albumin seed storage protein precursor
from English walnut (Juglans regia), a major food allergen J
Allergy Clin Immunol 101:807-14
-
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving
the sensitivity of progressive multiple sequence alignment through sequence
weighting, position-specific gap penalties and weight matrix choiceNucleic
Acids Res 22:4673-80
-
Vidal C, Díaz C, Sáez A, Rodríguez M,
Iglesias A (1991) Anaphylaxis to mustard Postgrad Med J 67:404
-
Villalba M, Monsalve RI, Batanero E, Ledesma A, González
E, Huecas S, Tejera ML, Palomares O, Cuesta I, Rodríguez R (2000)
Cloning
and expression of BnI, a 2S albumin from Brassica napus seeds with
allergenic properties Allergy 55:128 (abstract)
-
Widstrom L, Johansson SG (1986) IgE-mediated anaphylaxis
to mustard Acta Derm Venereol 66:70-1
-
Wirtz KW (1991) Phospholipid transfer proteins
Annu Rev Biochem 60:73-99
-
Youle RJ, Huang AH (1979) Albumin storage protein and
allergens in cottonseeds J Agric Food Chem 27:500-3
[Summary]
[Abbreveations]
copyright ©
2001 by matthias besler - ONLINE PUBLISHER
home:www.food-allergens.de
