Tanabe: Identification of Wheat
Allergens
Internet Symposium on Food Allergens
3(4):163-70 (2001) [http://www.food-allergens.de]
Wheat seeds are composed of
four protein classes, such as 1) water-soluble albumins, 2) salt-soluble
globulins, 3) ethanol-soluble gliadins, and 4) urea, detergent, or KOH-soluble
glutenins. Wheat gluten is a complex of gliadins
and glutenins. Gluten is the elastic rubbery protein that binds the dough
in breads and other bakery products.
Hypersensitivity responses
to wheat have long been an important public health problem. The adverse
reactions to wheat flour develop three different phenomena, enteropathy
(diarrhea), asthma and atopic dermatitis.
Gluten-sensitive enteropathy
is called "celiac disease", and is caused by ingestion of the gliadin fraction
(Sturgess et al. 1994). The reported prevalence of this disease is 1:300
to 1:1000 in European countries (Troncone et al. 1992). The amino acid
sequences responsible for the disease have been characterized, and the
minimum epitope structures were found to be Pro-Ser-Gln-Gln and Gln-Gln-Gln-Pro
(Sturgess et al. 1994). Although the disease is mediated by T-lymphocyte-driven
immunological activation in the gastrointestinal mucosa (Trier 1991, Marsh
1992, Troncone et al. 1996), the levels of total and wheat-specific IgE
antibodies of celiac patients are usually not elevated (Mietens et al.
1971, Hodgson et al. 1976, Bahna et al. 1980). Therefore, in the general
context of considering "allergy" synonymous with IgE-mediated hypersensitivity,
celiac disease should not be classified as an allergic disorder (Bahna
1996).
The inhalation of wheat
flour also often causes baker’s asthma (Amano et al. 1998), a typical occupational
allergic disease that has been known since ancient Roman times. Extensive
studies identified some proteins as allergens associated with asthma. Among
them, alpha-amylase inhibitors (AI) from the globulin fraction were identified
as major allergens (Gómez et al. 1990, Sánchez-Monge et al.
1992, Armentia et al. 1993, Amano et al. 1998). The IgE-binding epitope
structures of an AI (known as the 0.28 wheat AI) were determined (Walsh
& Howden 1989), and, acyl-CoA oxidase (Posch et al. 1995, Weiss et
al. 1997), peroxidase (Sánchez-Monge et al. 1997), and fructose-bisphosphate
aldolase (Weiss et al. 1997) were identified as other allergens.
The
other wheat-associated phenomenon is skin
inflammation, atopic dermatitis, that develops shortly after cereal-based
products are ingested, usually resulting in eruption and itching. Wheat
allergens associated with atopic dermatitis are so heterogeneous that many
attempts had been made internationally to identify them. However, little
information had been available on the molecular structure of major allergens
associated with atopic dermatitis when our group started to carry out a
systematic experiment (Varjonen et al. 1994, Varjonen et al. 1995, Watanabe
et al. 1995, Tanabe et al. 1996). Afterwards, some groups identified several
more wheat allergens (Sandiford et al. 1997, Kusaba-Nakayama et al. 2000,
Sander et al. 2001, Takizawa et al. 2001).
In our first experiment,
wheat flour proteins were divided into salt-soluble and salt-insoluble
(gluten) fractions (Table 1). The allergenicity of each fraction was evaluated
by means of enzyme-linked immunosorbent assay (ELISA), using sera of atopic
patients allergic to wheat; most patients were found to be sensitive to
gluten. Thus, we first tried to determine a major epitope structure of
gluten responsible for atopic dermatitis (Watanabe et al. 1995).
The
second approach aimed at identifying IgE-binding wheat proteins containing
carbohydrate moieties (Watanabe et al. 2001), since glycosylated subunits
of the alpha-amylase-inhibitor family were shown to have enhanced IgE-binding
capacity (Sánchez-Monge et al. 1992).
In
a further investigation we sought to evaluate the IgE-binding properties
of the polysaccharide fraction of wheat flour (Tanabe et
al. 2000).
Table 1: Allergenicities of salt-soluble and -insoluble (gluten)
fractions of wheat flour. Wheat flour proteins were divided
into salt-soluble and gluten fractions in the usual manner, and subjected
to ELISA. IgE-binding is expressed in arbitrary units based on the
absorbance at 490 nm.
|
Type of patient
|
Patient No.
|
Salt-soluble Fraction
|
Gluten Fraction
|
|
Sensitive to salt-soluble fraction
|
1
2
3
|
0.18
0.15
>2.0
|
<0.05
<0.05
0.07
|
|
Sensitive to gluten fraction
|
4
5
|
<0.05
0.05
|
>2.0
0.23
|
|
Sensitive to both fractions
|
6
7
8
9
|
0.16
>2.0
>2.0
>2.0
|
0.20
>2.0
>2.0
>2.0
|
1 IgE-BINDING TO AMINO ACID SEQUENCE
BASED STRUCTURES OF GLUTENIN
1.1 IgE-BINDING OF CHYMOTRYPTIC
PEPTIDES FROM LOW-MOLECULAR-MASS GLUTENIN
Since gluten was insoluble in aqueous media, it
was hydrolyzed with alpha-chymotrypsin to obtain soluble peptide fragments
with allergenicity. Food allergens are often characterized by their high
stability against digestive enzymes, with their epitope structures remaining
unchanged (Taylor et al. 1987). Thus digested peptides derived from the
gluten fraction were expected to be still capable of IgE-binding. The
resulting hydrolytic reaction product was centrifuged, and the supernatant
was subjected to gel filtration and reversed-phase HPLC. The allergenicity
of the fractionated elute was evaluated by ELISA, and the peak with the
highest allergenicity was subjected to a primary structure determination.
The primary structure of the purified compound was a 30-mer peptide
and determined to be (Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe)4.
This allergenic peptide showed high similarities (almost 90%) to low-molecular-mass
glutenin precursors (Pitts et al. 1988, Colot et al. 1989). Therefore,
we concluded the peptide originated from low-molecular-mass glutenin. Similarities
of about 70% were also obtained between the sequence of the allergenic
peptide and those of the low-molecular-mass glutenin precursors from durum
wheat (Cassidy & Dvorak 1990, D'Ovidio et al. 1992.). Surprisingly,
a high degree of similarity (53.6%) was also found between the allergenic
peptide and a Saccharomyces cerevisiae protein (Rasmussen 1994).
Thus, it should be noted that wheat allergic patients are also suspected
to be sensitive to yeast used for bread making.
The repeated sequence in allergenic peptides such as (Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe)4
may be favourable for cross-linking IgE antibodies and triggering the release
of chemical mediators from mast cells in our body. There exists a very
famous allergen, that is cod allergen (allergen M) which contains three
homologous IgE-binding tetrapeptides in the residues 41-64 (Elsayed et
al. 1982).
1.2 ANALYSIS OF IgE-BINDING EPITOPES
WITH SYNTHETIC PEPTIDES
In order to identify IgE-binding epitopes on the
30-mer amino acid sequence, peptides listed in Table 2 were synthesized
according to the solid phase method. The N-terminal amino acid of each
peptide was acetylated to mimic the condition under which each peptide
existed in intact form. The allergenicity of each peptide was evaluated
by ELISA (Tanabe et al. 1996). As shown in Table 2-A, (Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe)4,
(Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe)2, and Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe
bound to IgE almost equally. There was no difference between the relative
ELISA values of Ser-(Gln)4-Pro-Pro-Phe and Ser-(Gln)3-Pro-Pro-Phe.
These data suggest that the Ser-Gln-Gln-Gln-Pro-Pro-Phe motif is involved
in binding to IgE antibodies.
To examine which amino acid residues in the motif are essential for
binding to IgE, we replaced each constituent amino acid residue by Gly.
When any of the asterisked amino acid residues in the sequence Ser-Gln*-Gln-Gln-Pro*-Pro*-Phe
was replaced, the ELISA value dropped below the limit of detection (Table
2-B). These amino acid residues are therefore thought to be indispensable
for IgE-binding. Tables 2-B and C also show that Gln-Gln-Gln-Pro-Pro, which
lacks the N- and C-terminals of Ser-Gln-Gln-Gln-Pro-Pro-Phe, gave an ELISA
value equal to that obtained with the full peptide.
We further examined which amino acid residues of Gln-Gln-Gln-Pro-Pro
are essential for binding to IgE (Table 2-C) and found that the N-terminal
glutamine residue and the two proline residues are essential. It was thus
concluded that the IgE-binding epitope of the allergenic peptide comprised
Gln-X-Y-Pro-Pro, where X and Y were replaceable amino acid residues. Indeed
the inhibition ELISA assay showed that Ac-Gln-Gln-Gln-Pro-Pro bound to
wheat-specific IgE in the serum of patients. To analyze the binding between
Ac-Gln-Gln-Gln-Pro-Pro and IgE antibody, Fukushi et al. (1998) made the
first NMR analysis of Ac-Gln-Gln-Gln-Pro-Pro. Their data showed that the
configurations of the amide bonds of the peptide backbone were all-trans.
As in Table 2-C, the ELISA value obtained with Gln-Gly-Gln-Pro-Pro was
lower by almost 30% than that obtained with Gln-Gln-Gln-Pro-Pro, and the
value with non-acetylated Gln-Gln-Gln-Pro-Pro was almost half of that with
acetylated Gln-Gln-Gln-Pro-Pro. From these data, the second glutamine residue
of Gln-Gln-Gln-Pro-Pro and acetylation of the N-terminal amino group are
both advantageous for binding to IgE.
Also, recombinant low-molecular-mass glutenin, which contained many
Gln-Gln-Gln-Pro-Pro motifs, were expressed in Escherichia coli by
a pET vector system and confirmed its IgE-binding ability (Maruyama et
al. 1998).
Table 2: IgE-binding abilities of synthetic peptides. Peptides
were synthesized according to the solid phase method. The peptide-bound
multipins in a solid state were subjected to ELISA using sera of wheat
allergic patients. Amino acids are denoted in the single-letter code. (Tanabe
et al. 1996)
| (A) Peptide |
Relative ELISA value |
| Ac-SQQQQPPF SQQQPPF SQQQQPPF SQQQPPF |
1.0 |
| Ac-SQQQQPPF SQQQPPF |
1.1 |
| Ac-SQQQQPPF |
1.1 |
| Ac-SQQQPPF |
1.0 |
| (B) Peptide |
Relative ELISA value |
| Ac-GQQQPPF |
1.1 |
| Ac-SGQQPPF |
nd |
| Ac-SQGQPPF |
0.8 |
| Ac-SQQGPPF |
1.0 |
| Ac-SQQQGPF |
nd |
| Ac-SQQQPGF |
nd |
| Ac-SQQQPPG |
0.9 |
| (C) Peptide |
Relative ELISA value |
| Ac-QQQPP |
0.9 |
| Ac-GQQPP |
nd |
| Ac-QGQPP |
0.7 |
| Ac-QQGPP |
1.0 |
| Ac-QQQGP |
nd |
| Ac-QQQPG |
nd |
| QQQPP |
0.6 |
2 IgE-BINDING TO STRUCTURES
CONTAINING ASN-LINKED GLYCOCHAINS
Wheat alpha-amylase inhibitors (AIs) have been studied as allergens
for over 20 years. There is a family of wheat AIs with a number of differing
monomeric, dimeric and tetrameric proteins. As described above, IgE-binding
epitope structures of AI 0.28 have already been determined on the amino
acid sequence (Walsh & Howden 1989). Moreover, a glycan moeity of one
subunit from the tetrameric AI (CM16) was also capable
of IgE-binding from sera of patients with baker’s asthma (Sánchez-Monge
et al. 1992). Although, other AIs, such as AI 0.19, AI 0.28, and AI 0.53,
were also reported as allergens, involvement of glycans in allergic responses
has not been fully proven. As for the AI, James et al. (1997) showed that
AI was an allergen for both asthma and wheat allergy.
In addition, Asn-linked glycochains have received recent attention in
the studies on cross-reactivity between pollen, insects, and food allergens
(Batanero et al. 1996, Garcia-Casado et al. 1996). Garcia-Casado et al.
(1996) reported that the presence of a beta-1,2-xylosyl residue, which
was attached to the beta-linked mannose of the glycochain core in bromelain
and peroxidase, constituted an IgE-reactive determinant. Indeed, we previously
reported that patients sensitive to salt-soluble fraction of wheat flour
cross-reacted to bromelain (Tanabe et al. 1997). We thus examined IgE-binding
glycoproteins in wheat flour and clarified whether any new glycoprotein
occurred or not (Watanabe et al. 2001).
| Wheat flour was extracted with 10 mM sodium dihydrogenphosphate followed
by addition of ammonium sulfate to 50% saturation at pH 7.0. The precipitate
was dialyzed against running water, and then dissolved with 10 mM acetate
buffer (pH 4.5) containing 0.5 M NaCl. The solution was submitted to Carboxymethyl-(CM)-cellulose
and DEAE-cellulose column chromatography. The IgE-binding crude fraction
thus obtained was lyophilized. SDS-PAGE was carried out using a 7.5% gel,
and proteins in the gel were electrotransferred onto a PVDF membrane. The
same procedure was repeated three times. One of the membranes was immunoassayed
using sera of wheat-sensitive allergic patients (Figure 1, lane A). Another
membrane was submitted to immunodetection with a rabbit anti-HRP (horse
radish peroxidase) as a primary antibody, which recognizes peroxidase type
N-linked glycochains (Batanero et al. 1996) (lane B). The other membrane
was stained non-specifically
with coomassie
blue (CBB R-250) (lane C). There was one unknown IgE-binding protein in
all three lanes detected at about 60 kDa (asterisked band). The protein
reacted with the anti-peroxidase antibody (lane B), indicating that it
contained N-linked glycochain(s). The reactivity of the glycan moiety in
the 60 kDa allergen is under investigation. Bands at about 40 kDa and 16
kDa are probably peroxidase (Sánchez-Monge et al. 1997) and AIs
(James et al. 1997, Sánchez-Monge et al. 1997), respectively. |
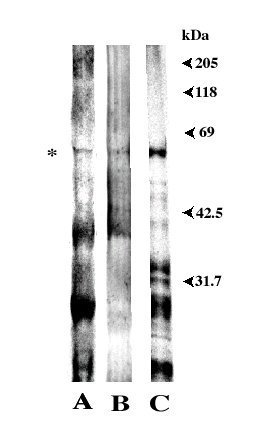
|
| Figure 1: Wheat flour proteins separated by SDS-PAGE.
(A) IgE reactivity of patients allergic to wheat flour, (B) immunoblot
with a rabbit anti-HRP (horse radish peroxidase) antibody which recognizes
peroxidase type N-linked glycochains, (C) CBB staining of wheat flour proteins.
(Watanabe et al. 2001) |
|
The N-terminal amino acid sequence of the asterisked band was determined
to be LDPDESEXVTRYFRIR. The 8th amino acid residue would be Asn to which
a glycochain attaches. The glycoprotein reacted both with the anti-horse
radish peroxidase IgG antibody and sera from several wheat-allergic patients.
The amino acid sequence similarity between the peptide fragment and other
naturally occurring proteins was checked using a sequence database. As
a result, no similarity was obtained between the sequence of the 60 kDa
glycoprotein and any other proteins including wheat allergens. Thus,
the glycoprotein was identified as a new wheat allergen.
3 IgE-BINDING TO POLYSACCHARIDE STRUCTURES:
MANNOGLUCAN
In the meantime, it remained unclear whether a non-proteinaceous constituent
in wheat also acts as an allergen. Unlike proteinaceous allergens, some
non-proteinaceous substances would be more stable in our body, possibly
acting as a remaining allergen to cause a longer-lasting allergic reaction.
Thus, the existence of such a non-proteinaceous allergen would explain
why wheat allergy is difficult to treat. Next, we aimed to isolate a polysaccharide
allergen from a water-soluble fraction of wheat flour and to clarify its
chemical structure and immunological properties (Tanabe et al. 2000).
The water-soluble fraction of wheat flour was first subjected to DEAE-cellulose
column chromatography to remove the proteinaceous substances. The unretained
fraction was then subjected to ConA-agarose affinity column chromatography
and gel filtration HPLC to isolate the fraction with
IgE-binding activity. The mean molecular mass was estimated to be approximately
50,000 kDa.
ConA is a specific adsorbent with an affinity
for mannose (Man)- and/or glucose (Glc)-containing polysaccharides and
glycoproteins. To clarify whether the IgE-binding compound consisted of
polysaccharide or glycoprotein, it was examined by IR spectrometry. The
IR spectrum of the allergenic compound suggested the presence of OH-groups,
with no characteristic absorption for amide groups being apparent. Therefore,
the compound appeared to consist mainly of polysaccharide. The polysaccharide
was hydrolyzed with 2 M TFA (trifluoroacetic acid), and the sugar composition
of the hydrolysate was analyzed by HPLC. The result revealed that the polysaccharide
consisted of Glc and Man in a molar ratio of 4.4 : 1, while no other common
sugars such as xylose, galactose, fucose, N-acetyl glucosamine, or N-acetyl
galactosamine were detected. Furthermore the polysaccharide allergen was
converted to oligosaccharides by hydrolysis with cellulase, suggesting
that the polysaccharide had beta-1,4-glycosidic linkages.
Judging from our detailed analysis, the polysaccharide
was a novel allergen with linear beta-1,4 linkages composed of Glc and
Man. While some studies have shown the presence of arabinoxylan and arabinogalactan
in water extracts of wheat flour, our report was the first that clearly
demonstrated the occurrence of mannoglucan in wheat flour (Tanabe et al.
2000).
The IgE-binding ability of wheat mannoglucan was
confirmed by inhibition ELISA. The water-soluble fraction of wheat flour
was coated on a microplate. Separately, patients’ sera were incubated with
wheat mannoglucan. After blocking unoccupied sites with bovine serum albumin
(BSA), the preincubated sera were used as the antibody for ELISA, and untreated
sera were used as controls. This procedure was followed by the addition
of biotinylated anti-human IgE, streptavidin-peroxidase conjugate, and
o-phenylenediamine. As a result, wheat mannoglucan inhibited antigen-antibody
binding by approximately 30% in all (four) patients allergic to the water-soluble
fraction of wheat flour.
While the orally administered mannoglucan allergen would be excreted
because of its indigestible nature, it could be absorbed by the inhalation
of wheat flour. In this case, it would not be degraded, and would remain
longer in the body as a remaining allergen. This would be the probable
reason why patients sensitive to the water-soluble fraction of wheat flour
are found to possess mannoglucan-specific IgE antibodies.
PERSPECTIVES
As I described, there are three classes of allergens in wheat flour;
1) proteins such as alpha-amylase inhibitors, low-molecular-mass glutenin,
acyl-CoA oxidase, peroxidase, fructose-bisphosphate aldolase, and so on,
2) Asn-linked glycochains in alpha-amylase inhibitor, peroxidase, and newly
found 60 kDa protein (however it should be noted that the reactivity of
the glycan moiety in these glycoprotein has not been fully proven), and
3) polysaccharide (mannoglucan). The knowledge of allergens will contribute
to the countermeasures against the worldwide social problem, intractable
wheat allergy. For example, hypoallergenic rice and wheat flour have been
produced for patients by our group. Such products should be of great benefit
to patients as was reported for hypoallergenic rice in Nature with
the title "Japan explores the boundary between food and medicine" (Swinbanks
& O’Brien 1993).
Moreover, recent reports indicate that strategies aiming specific immunotherapy
at the level of specific T cells are promising (Secrist et al. 1993, Bellinghausen
et al. 1997, Ebner et al. 1997). We aim at the identification
of T cell epitope structures of food allergens.
REFERENCES
-
Amano M, Ogawa H,
Kojima K, Kamidaira T, Suetsugu S, Yoshihama M, Satoh T, Samejima T, Matsumoto
I (1998) Identification of the major allergens in wheat flour responsible
for baker’s asthma Biochem J 330:1229-34
-
Armentia A, Sánchez-Monge
R, Gómez L, Barber D, Salcedo G (1993) In vivo allergenic
activities of eleven purified members of a major allergen family from wheat
and barley flour Clin Exp Allergy 23:410-5
-
Bahna SL, Tateno K, Heiner DC (1980) Elevated
IgD antibodies to wheat in celiac disease Ann Allergy 44:146-51
-
Bahna SL (1996) Celiac disease: A food
allergy? Contra! in "Highlights in Food Allergy"
(Wuthrich B & Ortolani C eds) pp 211-5 Karger, Basel
-
Batanero E, Villalba M, Monsalve RI, Rodriguez
R (1996) Cross-reactivity between the major allergen from olive pollen
and unrelated glycoproteins: evidence of an epitope in the glycan moiety
of the allergen J Allergy Clin Immunol 97:1264-71
-
Bellinghausen I,
Metz G, Enk A H, Christmann S, Knop J, Saloga J (1997) Insect venom
immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift,
and changes surface marker expression in venom-allergic subjects Eur
J Immunol 27 1131-9
-
Cassidy BG, Dvorak
J (1990)
Accession no.: S08683 EMBL Data Library
-
Colot V, Bartles
D, Thompson R, Flavell R (1989) Molecular characterization of an active
wheat LMW glutenin gene and its relation to other wheat and barley prolamin
genes
Mol Gen Genet 216:81-90
-
D'Ovidio R, Tanzarella
O A, Porceddu E (1992) Nucleotide sequence of a low-molecular-weight
glutenin from Triticum durum Plant Mol Biol 18:781-4
-
Ebner C, Siemann
U, Bohle B, Willheim M, Wiedermann U, Schenk S, Klotz F, Ebner H, Kraft
D, Scheiner O (1997) Immunological changes during specific immunotherapy
of grass pollen allergy: reduced lymphoproliferative responses to allergen
and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major
grass pollen allergen Clin Exp Allergy 27:1007-15
-
Elsayed S, Sornes
S, Apold J, Vik H, Florvaag E (1982) The immunological reactivity of
the three homologous repetitive tetrapeptides in the region 41-64 of allergen
M from cod Scand J Immunol 16:77-82
-
Fukushi E, Tanabe
S, Watanabe M, Kawabata J (1998) NMR analysis of a model pentapeptide,
acetyl-Gln-Gln-Gln-Pro-Pro, as an epitope of wheat allergen Magn
Reson Chem 36 741-6
-
Garcia-Casado G,Sanchez-Monge R, Chrispeels
MJ, Armentia A, Salcedo G, Gomez L (1996) Role of complex asparagine-linked
glycans in the allergenicity of plant glycoproteins Glycobiology6:471-7
-
Gómez L,
Martin E, Hernandez D, Sánchez-Monge R, Barber D, del Pozo V, De
Andres B, Armentia A, Lahoz C, Salcedo G, Palomino P (1990) Members
of the alpha-amylase inhibitors family from wheat endosperm are major allergens
associated with baker's asthma FEBS Lett 261:85-8
-
Hodgson HJ, Davies RJ, Gent AE (1976)
Atopic
disorders and adult coeliac disease Lancet 1(951):15-117
-
James JM, Sixbey JP, Helm RM, Bannon GA,
Burks AW (1997) Wheat alpha-amylase inhibitor: a second route of allergic
sensitization J Allergy Clin Immunol 99 239-244
-
Kusaba-Nakayama
M, Iwamoto M, Shibata R, Sato M, Imaizumi K (2000) CM3, one of the wheat
alpha-amylase inhibitor subunits, and binding of IgE in sera from Japanese
with atopic dermatitis related to wheat Food Chem Toxicol 38
179-85
-
Marsh MN (1992) Gluten, major histocompatibility
complex, and the small intestine Gastroenterology 102:330-54
-
Maruyama N, Ichise
K, Katsube T, Kishimoto T, Kawase S, Matsumura Y, Takeuchi Y, Sawada T,
Utsumi S (1998) Identification of major wheat allergens by means of
the Escherichia coli expression system Eur J Biochem255 739-45Mietens
C, Johansson SGO, Bennich H (1971) Serum concentration of immunoglobulins
with special concentration of IgE in celiac disease Klin Wochenschr49:256-60
-
Pitts E G, Rafalski
J A, Hedgcoth C (1988) Nucleotide sequence and encoded amino acid sequence
of a genomic gene region for a low molecular weight glutenin Nucl
Acids Res 16:11376
-
Posch A, Weiss W,
Wheeler C, Dunn MJ, Gorg A (1995) Sequence analysis of wheat grain allergens
separated by two-dimensional electrophoresis with immobilized pH gradientsElectrophoresis16:1115-9
-
Rasmussen S W (1994)
Sequence
of a 286 kb region of yeast chromosome XI includes the FBA1 and TOA2 genes,
an open reading frame (ORF) similar to a translationally controlled tumour
protein, one ORF containing motifs also found in plant storage proteins
and 13 ORFs with weak or no homology to known proteins
Yeast 10:S63-68
-
Sánchez-Monge
R, Gómez L, Barber D, Lopez-Otin C, Armentia A, Salcedo G (1992)
Wheat
and barley allergens associated with baker's asthma glycosylated subunits
of the alpha-amylase-inhibitor family have enhanced IgE-binding capacityBiochem
J 281:401-5
-
Sánchez-Monge
R, Garcia-Casado G, Lopez-Otin C, Armentia A, Salcedo G (1997) Wheat
flour peroxidase is a prominent allergen associated with baker's asthmaClin
Exp Allergy 27:1130-37
-
Sander I, Flagge
A, Merget R, Halder TM, Meyer HE, Baur X (2001) Identification of wheat
flour allergens by means of two-dimensional immunoblots J Allergy
Clin Immunol 107:907-13
-
Sandiford CP, Tatham
AS, Fido R, Welch JA, Jones MG, Tee RD, Shewry PR, Newman Taylor AJ (1997)
Identification
of the major water/salt insoluble wheat proteins involved in cereal hypersensitivityClin
Exp Allergy 27:1120-9
-
Secrist H, Chelen
C J, Wen Y, Marshall J D, Umetsu D T (1993) Allergen immunotherapy decreases
interleukin 4 production in CD4+ T cells from allergic individualsJ
Exp Med 178:2123-30
-
Sturgess R, Day P, Ellis HJ, Gjertsen
HA, Kontakou M, Ciclitira PJ (1994) Wheat peptide challenge in coeliac
disease Lancet343:758-61
-
Swinbanks D, O’Brien
J (1993)
Japan explores the boundary between food and medicine Nature364:180
-
Takizawa T, Arakawa
H, Tokuyama K, Morikawa A (2001) Identification of allergen fractions
of wheat flour responsible for anaphylactic reactions to wheat products
in infants and young children Int Arch Allergy Immunol 125:51-6
-
Tanabe S, Arai S, Yanagihara Y, Mita H,
Takahashi K, Watanabe M (1996) A major wheat allergen has a Gln-Gln-Gln-Pro-Pro
motif identified as an IgE-binding epitope Biochem Biophys Res Commun219:290-3
-
Tanabe S, Tesaki S, Watanabe M, Yanagihara
Y (1997)
Cross-reactivity between bromelain and soluble fraction from
wheat flourJpn J Allergol 46:1170-3 (in Japanese)
-
Tanabe S, Watanabe J, Oyama K,
Fukushi E, Kawabata J, Arai S, Nakajima T, Watanabe
M (2000) Isolation and characterization of a novel polysaccharide as
a possible allergen occurring in wheat flour Biosci Biotechnol Biochem
64:1675-80
-
Taylor SL, Lemanske
Jr RF, Bush RK, Busse WW (1987) Food allergens: structure and immunologic
propertiesAnn Allergy 59:93-9
-
Trier JS (1991) Celiac sprue N
Engl J Med325:1709-19
-
Troncone R, Caputo N, Zibella A, Molitierno
G, Maiuri L, Auricchio S (1992) Coeliac disease: A common food intolerance
on an immunological basis in Common Food Intolerances 1: Epidemiology
of Coeliac disease (Auricchio S & Visakorpi JK, eds) pp 1-11, Karger,
Basel
-
Troncone R, Greco L, Auricchio S (1996)
Gluten-sensitive
enteropathy Pediatr Clin North Am 43:355-73
-
Varjonen E, Savolainen
J, Mattila L, Kalimo K (1994) IgE-binding components of wheat, rye,
barley and oats recognized by immunoblotting analysis with sera from adult
atopic dermatitis patients Clin Exp Allergy 24:481-9
-
Varjonen E, Vainio
E, Kalimo K, Juntunen-Backman K, Savolainen J (1995) Skin-prick test
and RAST responses to cereals in children with atopic dermatitis Characterization
of IgE-binding components in wheat and oats by an immunoblotting method
Clin
Exp Allergy 25:1100-7
-
Walsh BJ, Howden
ME (1989) A method for the detection of IgE binding sequences of allergens
based on a modification of epitope mapping J Immunol Methods121:275-80
-
Watanabe J, Tanabe
S, Sonoyama K, Kuroda M, Watanabe M (2001) An IgE reactive 60kDa glycoprotein
occurring in wheat flour Biosci Biotech Biochem65:2102-5
-
Watanabe M, Tanabe S, Suzuki T, Ikezawa
Z, Arai S (1995) Primary structure of an allergenic peptide occurring
in the chymotryptic hydrolysate of gluten Biosci Biotech Biochem
59:1596-7
-
Weiss W, Huber G,
Engel KH, Pethran A, Dunn MJ, Gooley AA, Gorg A (1997) Identification
and characterization of wheat grain albumin/globulin allergens Electrophoresis
18:826-33
[Summary]
[Abbreveations]
copyright © 2001 by matthias besler -
ONLINE PUBLISHER
home: www.food-allergens.de
