More than 20 % of the population in industrial countries suffer from allergies, especially from type I allergy. The allergic reaction is antigen specific and the structure of the allergen is involved in both the sensitization process and in the elicitation of symptoms. Therefore, the answer to the question why a certain antigen becomes an allergen must be related to its structural characteristics.
In order to study the structural impact of allergens, we focused on grass pollen allergens. Their antigenic repertoires are quite similar among different species and show cross-reactivities to a lot of foods such as tomato (De Martino et al. 1988, Petersen et al. 1996), kiwi (Pastorello et al. 1996), apple, carrot and celery (Halmepuro & Löwenstein 1985). The cross-reactivity is only in part due to profilins as demonstrated by Heiss et al. (1996) and Petersen et al. (1996).
Among the grass allergens, group I is particularly important with an IgE prevalence of 95 % (Freidhoff et al. 1986). These allergens are glycoproteins with a molecular mass of 30 - 40 kDa and are detectable in all grasses (Knox et al. 1989). According to Laffer et al. (1996) the group I components reveal a high sequence homology of up to 95%.
We intended to identify the IgE-reactive epitopes, since they are the
structural elements that finally cause the allergic attacks.
Therefore, they are the goals for diagnosis and specific immunotherapy.
Additionally, we tried to determine structural features that might be involved
in the sensitization process. This attempt should increase understanding
of the mechanism of the allergen specific immune reaction and probably
help to prevent allergy.
DETERMINATION OF IgE-REACTIVE EPITOPES
In order to identify the IgE-binding epitopes, the elucidation
of the complete primary structure is mandatory. We obtained the recombinant
group I allergens after screening the cDNA libraries of timothy grass (Phleum
pratense) and sweet velvet grass (Holcus lanatus) by use of
the monoclonal antibody IG 12 (Petersen et al. 1995a, Schramm et al. 1997).
Both allergens (Phl p 1 and Hol l 1) consist of 240 amino acids and reveal
a considerable degree of 90% sequence identity (Petersen et al. 1995a).
Complete rHol l 1 and its fragments were expressed as fusion proteins (containing
the maltose binding protein) in E. coli to perform an epitope mapping.
This method allowed the localization of IgE-binding epitopes on large fragments
and a stepwise limitation to the epitope size by lowering the peptide length.
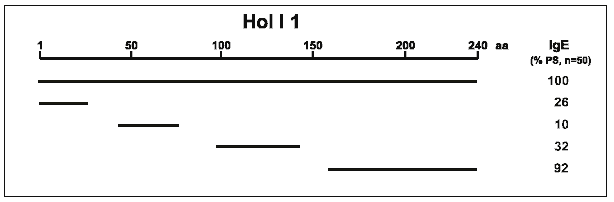
Figure 1 shows the data after analysing 50 patient sera by Western blotting.
The results indicate that at least four independent IgE-binding regions
exist on Hol l 1 (Schramm et al. 1997). One important epitope comprising
80 amino acids was located at the C-terminus. Smaller fragments of this
region did not show IgE reactivity and thus indicated a conformational
epitope.
 |
| Figure 1: IgE-epitope mapping on rHol l 1 (Schramm et al. 1997). The schematic map shows that there are at least 4 independent IgE-binding regions on the complete recombinant molecule. The number of IgE-reactive sera is given as percentage of all investigated sera. |
| Table 1: N-terminal sequences of natural and in E. coli expressed recombinant Phl p 1 determined by protein sequencing (Petersen et al. 1997a). nPhl p 1 bears two hydroxylated proline residues (Hyp) and one N-glycosylation site (?) as post-translationally modified amino acids. | ||||||||||||||||||||||||||||||||||||
|
To localize continuous epitopes precisely we (Petersen et al. 1998) used the pepscanning technique. Overlapping decapeptides were synthesized by the pin technology (Geysen et al. 1987) and analysed for IgE reactivity with individual patient sera. We determined one epitope at the N-terminus of Phl p 1 which was recognized by only a few sera. (The N-terminal structure is shown in Tab. 1). Ball et al. (1994) identified another sequential epitope in Phl p 1 harbouring the region of amino acids 101 - 115.
Phl p 1 bears several post-translational modifications (Petersen et al. 1995b, 1997a, 1997b, 1998). Since these modifications do not exist in recombinant proteins expressed in E. coli, we investigated the IgE reactivity of the natural molecules. The influence of disulfide bonds on the IgE binding capacity was examined by ELISA using 20 individual sera. While denaturation by 6 M guanidine hydrochloride did not cause any change, the IgE reactivity of reduced and carboxymethylated Phl p 1 generally was lowered by 25%. This emphasizes the impact of disulfide bridges on the allergenic structure of Phl p 1.
The carbohydrate structure of Phl p 1 with an alpha-1,3- bound fucose at the innermost N- acetylglucosamine was shown to be the essential configuration of an IgE-binding epitope (Petersen et al. 1996). IgE reactivity to glycans of similar structure was demonstrated for several allergens, but a mediator release due to carbohydrates has not been shown before. Instead of Phl p 1 (the complete carbohydrate structure has not been identified so far), we isolated N- linked glycopeptides from bromelain which show a similar carbohydrate structure to Phl p 1, and coupled them to BSA. The conjugates elicited histamine release from basophils of a patient sensitized to carbohydrate determinants. Since we were able to show that Phl p 1 partially forms dimers, we assume that such reactions can be caused by carbohydrate chains alone even in the absence of IgE against peptide epitopes.
Even small exchanges, e.g. post-translational hydroxylation of proline residues, can increase IgE reactivity (Petersen et al. 1998). According to protein sequencing data the amino acids in positions 5 and 8 were hydroxyproline residues (Tab. 1). We constructed synthetic decapeptides with proline and hydroxyproline residues, respectively, and investigated their IgE binding in an ELISA. The modified peptides revealed a 30% increased reactivity.
In summary, these results indicate that the protein conformation
and posttranslational modifications are involved in the generation of IgE-reactive
epitopes. Because of the diversity of the epitopes no single eliminations
seem to be promising to engineer safe immuno therapeutics. We (Petersen
et al. 1999) assume that in contrast to the Bet v 1 molecule (Vrtala et
al. 1997) it is not possible to destroy the allergenicity of Phl p 1 by
simply cleaving the allergen into two fragments. Due to the disulfide bonds
in Phl p 1, reduction will only decrease the IgE reactivity by about 25%.
This is in contrast to Der p 2, where site-directed mutagenesis of single
cysteine residues caused a 100fold decrease of IgE reactivity (Smith &
Chapman 1996). Thus, Phl p 1 is a very complex allergen and no single manipulation
will reduce its allergenicity considerably.
DETERMINATION OF THE PROTEIN FUNCTION
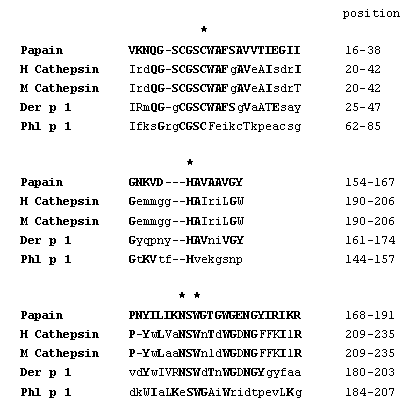
When we expressed Phl p 1 in the eukaryotic Pichia pastoris system, we observed a severe degradation of the recombinant protein. Detailed studies of Grobe et al. (1999) revealed that fragmentation was not caused by yeast enzymes but by rPhl p 1 itself. By APIZYM test (BioMérieux, Lyon, France), a screening test for enzymes, rPhl p 1 could be identified as a proteinase which is specific for basic amino acid residues. Identity of Phl p 1 as a cysteine proteinase was confirmed by experiments using specific substrates and inhibitors (Grobe et al. 1999). Preincubation of rPhl p 1 with an appropriate, cysteine protease activating buffer resulted in a detectable reactivity. Further indications that Phl p 1 is a C1 related cysteine protease were deduced from sequence alignments. Whereas the complete enzymes showed a homology of only about 10%, the three consensus regions forming the catalytic site reveal a considerable degree of structural similarity as depicted in Figure 2.
Except for the asparagine residue in the third domain, Phl p 1 fulfills the typical criteria for the classification as aC1 protease. Further molecular and biochemical similarities are the presence of a tryptophan-rich C-terminal region (Berti & Storer 1995), the presence of the active cysteine-histidine pair in the corresponding sequence motifs (Rawlings & Barrett 1994), the similar size of the proteins and their common cellular localization.
These results clearly indicate that Phl p 1 is an unactivated protease. Up to now nothing is known about the activation of nPhl p 1 in vivo.
| Figure 2: Comparison of C1 cysteine proteinases (papain,
human and mouse cathepsin, and Der p 1) in the three consensus motifs forming
the catalytic site. Important
amino acid residues are indicated by asterisks. Bold printed, capital letters
mark identical, capital letters mark similar amino acid residues to the
papain sequence.
|
 |
IMPLICATIONS OF Phl p 1 FOR THE PATHOMECHANISM OF THE TYPE I ALLERGY
As a proteolytic enzyme Phl p 1 might act on different levels to mediate the sensitization process. Here, we (Petersen et al. 1999) discuss assumptions, drawn from observations of other enzymes and/or allergens, especially Der p 1, which is also a C1 cysteine protease.
The activated Phl p 1 might increase the permeability of the nasal and lung mucosa and thus enable the allergen to reach the immune system (Fig. 3). This was shown for several other proteolytic enzymes (Herbert et al. 1995, Stewart et al. 1993).
In a second step the enzyme could effect T helper cells to cause a bias to TH2 (Fig. 3). Dudler et al. (1995) described IL-4 release from mouse mast cells by the active phospholipase A2 from bee venom in contrast to the inactive enzyme. Recently, Schulz et al. (1998) demonstrated a direct effect of Der p 1 on the IL-2 receptor of T helper cells. Cleavage of this receptor caused a lower proliferation of T cells and a decreased IFN-gamma secretion.
Finally, the active enzyme could directly influence plasma cells to increase IgE production (Fig. 3). This process is induced by enzymatical cleavage of CD23 (the low affinity Fc-epsilon-RII receptor) from B cells as was shown by Hewitt and Schulz (Hewitt et al. 1995, Schulz et al. 1997). Due to this, an important inhibitory signal for IgE synthesis is deleted (Sherr et al. 1989). Moreover, the CD23 fragment can interact with CD21 on B cells and provoke an induction of IgE synthesis (Aubry et al. 1992).
The CD23 cleavage can be inhibited by reagents e.g. alpha-antiprotease (Hewitt et al. 1995). Several cysteine protease inhibitors were identified in saliva and skin (Isemura et al. 1984, Jarvinen 1978). We assume that allergic patients might lack such protease inhibitors and they are therefore destined to become sensitized. Once an individual has been sensitized a wide variety of IgE-reactive epitopes can repetitively elicit allergic reactions.
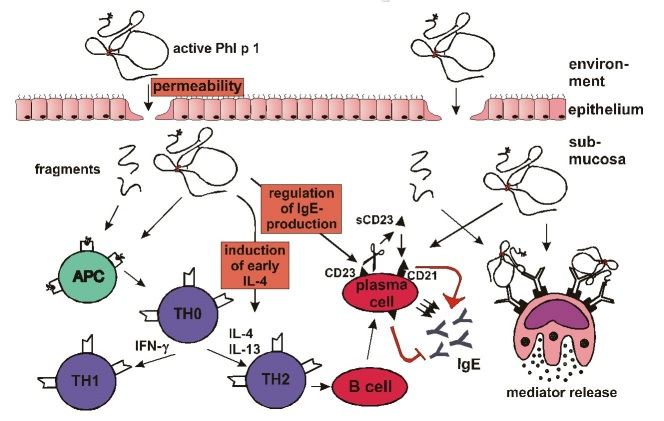 |
| Figure 3: Influence of Phl p 1 on the mechanism of the allergic reaction. (Details are described in the text.) |