*Including foods such as papaya, fig, passion fruit, Prunoideae fruits (peach, medlar, plum), potato, nuts and cereals.
U, urticaria; AE, angioedema; OAS, oral allergy syndrome.
It is well known that certain IgE-mediated sensitizations to aeroallergens are significantly associated with a variety of food hypersensitivities. These clinically very important - and at the same time intriguing - associated allergies are sometimes referred to as syndromes. Examples are the birch-pollen apple associated allergy, or the mugwort-celery spice syndrome (Fritsch et al. 1997). The immunopathological basis for these clinical associations is the cross-reactivity among antigens from different sources, due to molecular similarities among their epitopes. These cross-reactions, or recognition of different antigens by the same antibodies, are investigated in the laboratory by IgE-binding inhibition experiments (Vuitton 1997).
Some of the antigens responsible for these cross-reactivities have been recently identified. The characterization of Bet v 1 and its homologous - tree pollen group I allergens - has played a critical role in the understanding of the food-pollen allergy syndrome (Ipsen & Lowenstein 1983). They constitute a ubiquitous family of plan-defense proteins, belonging to one of the groups of pathogenesis-related proteins (PRP). These PRP can increase their expression in response to certain external factors, leading to the hypothesis that modern chemically-treated plants could be more allergenic. In a similar way, profilins are a group of proteins associated with the cytoskeleton system, highly conserved in the plant kingdom, which therefore act as panallergens (Valenta et al. 1992). In fact, associated sensitization among birch and mugwort pollens, apple and celery, seems to be based on the presence of Bet v1 homologous, profilins, and Art v 1, a major allergen in mugwort (Bauer et al. 1996).
At the same time, latex IgE-mediated hypersensitivity has been recognized as a very important international health problem during the last decade (Sussman & Beezhold 1995, Turjanmaa et al. 1996). The increase in frequency of latex allergy, the potential severity of the latex-induced reactions, and its presentation as an occupational disease in workers using gloves, are matters of great concern (Lagier et al. 1992, Carrillo et al. 1995). Several latex allergens have been identified, of which 10 have received an international nomenclature designation (Breiteneder & Scheiner 1998). Among them, prohevein or Hev b 6 is a chitin-binding protein with a molecular mass (Mr) of 20 kDa, which seems to be a major latex allergen (Alenius et al. 1995). The IgE-binding capacity of prohevein is mostly attributed to its N-terminal domain, known as hevein, with a Mr of 4.7 kDa.
 |
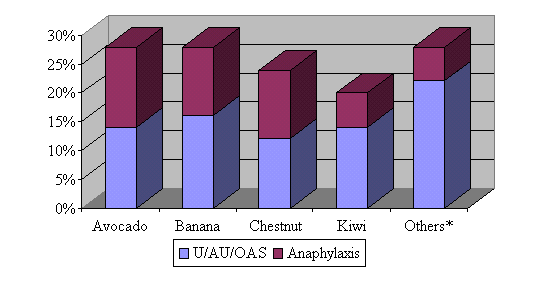
| Figure 1: Relative frequency and
clinical manifestations of food allergies in a group of 50 patients allergic
to latex
*Including foods such as papaya, fig, passion fruit, Prunoideae fruits (peach, medlar, plum), potato, nuts and cereals. U, urticaria; AE, angioedema; OAS, oral allergy syndrome. |
It was at the beginning of the 90s when a patient with latex and banana associated allergy was first described (MíRaihi et al. 1991). In this study, although there is no botanical relationship between latex and banana, cross-reactivity was demonstrated between them, through RAST-inhibition assays. Soon after, a patient allergic to latex, avocado and banana was reported (Lavaud et al. 1992). At the same time, 3 patients with latex-fruit associated allergy were described (Ceuppens et al. 1992). In 1993, there were some reports from Spain on patients with latex-chestnut hypersensitivity, and cross-reaction was demonstrated between them (Rodríguez et al. 1993, de Corres et al. 1993, Añíbarro et al. 1993).
It was in 1994 when the existence of a "latex-fruit syndrome" was proposed, based on the clinical observation of an unexpectedly high rate of fruit hypersensitivity in a group of 25 latex-allergic patients (Blanco et al. 1994). In this study, almost 50% of latex allergic patients showed hypersensitivity to one or more of these fruits. Approximately half of the reported episodes consisted of systemic anaphylactic reactions, thus showing the clinical relevance of these associated sensitizations. Implicated fruits were mainly chestnut, avocado and banana; but kiwi, papaya and other foods were also involved.
By 1997, 50 latex-allergic patients had been included in this study, of which 46% showed food hypersensitivities (Blanco 1997). This association was very significant (chi-square test p<0.0001) in comparison with a control group of patients paired for age, sex and atopy. Among these 50 patients, a total of 72 food symptomatic sensitizations were diagnosed. Foods responsible for the adverse reactions, as well as the clinical manifestations, are summarized in Figure 1. Banana and avocado hypersensitivities were the most frequent ones (28% of the 50 latex-allergic patients showed allergy to them), followed by chestnut (24%) and kiwi (20%).
With respect to their clinical manifestations, half the reactions consisted
of systemic anaphylaxis, and the other half varied among urticaria, angioedema
or oral allergy syndrome. Ten of our latex-allergic patients (20%) showed
simultaneous allergy to 3 or more foods, and in half of them the number
of food sensitivities increased with time. By clinical history, latex allergy
preceded food hypersensitivity in 12 patients, a simultaneous food- and
latex-allergy onset occurred in 6 cases, and food allergy was present before
latex allergy in the remaining 5 patients (Blanco 1997).
THE CONFIRMATION OF THE EXISTENCE OF
THE LATEX-FRUIT SYNDROME:
DATA FROM OTHER COUNTRIES
Although there is no taxonomical relationship among the various vegetal species implicated in the latex-fruit syndrome, its existence has been fully confirmed by other authors from different countries. In a Finish study, 52% of 31 latex-allergic patients reported symptoms after eating bananas, and banana prick by prick test (PPT) showed positive results in 35% of them (Mäkinen-Kiljunen 1994). Other authors found a 58% rate of associated fruit allergy to banana and/or avocado among 17 latex-allergic patients (Lavaud et al. 1995). In another study, 50% of 16 latex allergic patients reported symptoms after eating bananas, and banana PPT was positive in 36% of 14 patients (Delbourg et al. 1996). In a study from Canada, 36% of 47 latex-allergic patients manifested a clinical allergy to at least one food, banana, potato, and avocado being most frequently implicated (Beezhold et al. 1996). Statistical comparisons with a control group demonstrated significant associated sensitizations among latex and several foods, including avocado, potato, banana, tomato, chestnut, and kiwi.
In a larger series of latex-allergic patients, 42.6% of 136 patients reported adverse reactions after ingestion of a wide range of fruits, kiwi and banana being the most frequent (Brehler et al. 1997). Cross-reacting IgE antibodies recognizing latex and fruit allergens (avocado, banana, chestnut, kiwi, papaya, passion fruit, fig, melon, mango, pineapple, peach, and tomato) were demonstrated by RAST-inhibition tests. More recently, 49 potential allergic reactions to foods were identified in 29 (21%) of 137 patients with latex allergy from the USA (Kim & Hussain 1999). Foods responsible for these reactions included not only banana, avocado, and kiwi, but also shellfish and fish.
A comparison among our patients and the above mentioned
studies on latex-food allergy reported from other countries lead to the
following observations:
| 1. | The rate of latex allergic patients who show associated food allergy varied from 21% (Kim & Hussain 1999) to 58% (Lavaud et al. 1995) among the studies considered (Table 1). This variation could be explained by differences in diagnostic criteria, both for latex allergy and for food hypersensitivity. In this context, criteria for diagnosing latex allergy are not standardized, and a gold standard is not available, and therefore latex allergic patients are not similarly selected. Furthermore, food oral challenge tests have not been performed in these patients, leading to a probable over-diagnosis of food allergies. However, differences in food consumption habits could also play a role (see below). In any case, as usually observed when dealing with food allergy, the rate of food sensitizations among latex-allergic patients could be considerable higher, many of them being asymptomatic (Beezhold et al. 1996). |
| 2. | As previously suspected (Blanco et al. 1994), the type and proportion
of food sensitivities associated to latex allergy vary
among these studies (Figure 2). This fact may be explained by differences in the nutritional habits among countries (Brehler at al. 1997). As an example, chestnut and avocado allergy are less frequently diagnosed in Germany than in Spain, probably because these foods are consumed less in the former. However, in this context it is difficult to explain the high rate of potato allergy found in Canada (Beezhold et al. 1996), or of shellfish allergy found in the USA (Kim & Hussain 1999), because these findings are not described by other authors. Table 2 tries to summarize the food hypersensitivities associated with latex-allergy. |
| 3. | In the same way, the rate of food anaphylactic reactions varied among the mentioned studies, from 50% (Blanco 1997) to less than 5% (Brehler et al. 1997) of recorded food adverse reactions. Again, differences in diagnostic criteria and food consumption habits could explain these figures. In fact, certain foods seem to be more prone to induce systemic anaphylaxis, such as banana, avocado, chestnut, and kiwi (Blanco et al 1994, Beezhold et al. 1996). Other foods which are not very often associated with latex allergy may also induce anaphylactic reactions, as for example fig, papaya, and tomato. Meanwhile, foods such as potato usually induce mild local reactions (Beezhold et al. 1996). As frequently described with latex allergy, a systemic anaphylaxis could be the initial manifestation of the food hypersensitivity. |
| 4. | Although latex allergy precedes food hypersensitivity in most patients, the opposite is also seen in some subjects, as we have mentioned above. In agreement with our results, other authors found that the onset of latex allergy preceded food allergy in 12 out of 29 patients, in 11 cases food allergy was present before, and in 1 patient there was a simultaneous onset (Kim & Hussain 1999). In the same way, and as we have observed in half our patients, other studies have also shown that in many cases the spectrum of food allergies may increase with time. For example, in a series of 29 patients, 5 developed new food hypersensitivities after acquiring latex allergy (Kim & Hussain 1999). |
Table 1: Rate of food hypersensitivity among latex-allergic patients
| Reference | Country | No. of latex-allergic patients | % of food sensitization / allergy
(diagnostic criteria) |
| Blanco et al. 1994 | Spain | 25 | 52% (history + PPT) |
| Mäkinen-Kiljunen 1994 | Finland | 31 | 52% (history)
35% (PPT) |
| Lavaud et al. 1995 | France | 17 | 58% (history + SPT) |
| Delbourg et al. 1996 | France | 16 | 50% (history)
36% (SPT) |
| Beezhold et al. 1996 | Canada | 47 | 36% (history + SPT)
70% (SPT) |
| Blanco 1997 | Spain | 50 | 46% (history + PPT) |
| Brehler et al. 1997 | Germany | 136 | 43% (history)
69% (IgE) 14% (history + IgE) |
| Kim & Hussain 1999 | USA | 137 | 21% (history) |
| Group | Definition | Foods |
| I | Frequent and significant associations | Banana, avocado, kiwi, chestnut |
| II | Significant associations, but only described in certain studies | Potato, shellfish |
| III | Common associations, but number of cases not enough to reach significant levels | Papaya, tomato, pineapple, passion fruit, mango, fig, nuts (almond, hazelnut), stone fruits (peach, cherry, apricot), melon, apple |
| IV | Less common associations | Guava, fish, carrot, pear, strawberry, peanut, pepper, grape |
| V | Reported associations | Coconut, oregano, sage, dill, condurango bark, milk, spinach, beet, loquat. |
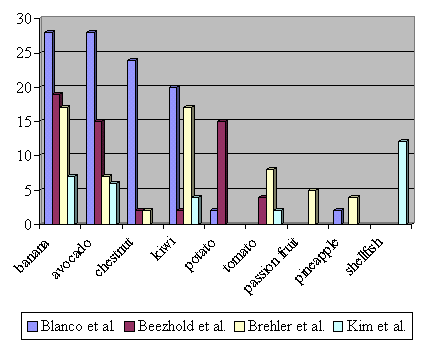 |
| Figure 2: Food hypersensitivities associated with latex allergy: a comparison of several studies. (Frequencies in % of latex allergic patients) |
THE OTHER POINT OF VIEW: LATEX SENSITIZATION AMONG FOOD-ALLERGIC PATIENTS
In a study on avocado hypersensitivity, we have observed that 10 out of 17 avocado allergic patients showed associated allergy to latex (Blanco et al. 1994). Soon after, in an epidemiological study on greenhouse workers, we have demonstrated that certain food hypersensitivities - avocado, chestnut, banana, and almond - increased the risk of latex allergy 24 times (Carrillo et al. 1995). In the same way, some recent studies try to investigate the prevalence of latex allergy among patients diagnosed with fruit allergy. In one of them, six out of 57 fruit-allergic patients were found to suffer from clinically relevant latex allergy, although the rate of asymptomatic latex sensitization was considerably higher (García et al. 1998). In all these patients, clinical symptoms to fruits preceded the history of latex allergy. The fruits most commonly associated to latex allergy were banana, peach, and melon. Moreover, all the patients who had had problems with foods such as banana, avocado, chestnut, or tomato, were sensitized to latex.
In another study, 2 out of 29 patients allergic to fruits
or vegetables who lived in an area without birch trees were clinically
allergic to latex (Díez-Gómez et al. 1999). As expected,
patients with allergy to plant-derived food and associated pollinosis showed
a high frequency of IgE reactivity to profilins. Taking into consideration
that one of latex allergens is a profilin (Vallier at al. 1995), this fact
may explain the finding of positive serum IgE determinations to latex and
birch pollen in patients allergic to plant-derived foods, due to the presence
of cross-reactive epitopes (Díez-Gómez et al. 1999). In a
very recent study, twice as many positive PPT to foods were found in patients
with pollinosis, whether or not they were allergic to latex, as were found
in patients allergic to latex but not to pollens (Levy et al. 2000). Latex
allergy was associated with avocado or banana hypersensitivity, whereas
pollinosis was associated with allergy to apple, peach or celery. These
results suggest that concomitant allergy to pollen is an important factor
in the determination of which plant-derived foods sensitizes latex-allergic
patients (Levy et al. 2000).
IDENTIFICATION OF THE ALLLERGENS RESPONSIBLE FOR THE LATEX-FRUIT SYNDROME
Cross-reactivity among latex and various fruits has been fully demonstrated by RAST inhibition (Ross et al. 1992, Blanco et al. 1994, Mäkinen-Kiljunen 1994, Ahlroth et al. 1995, Brehler et al. 1997), and several common antigens identified by immunoblot inhibition experiments (Alenius et al. 1996, Möller et al. 1998). Interestingly, a 30 kDa antigen common to latex, avocado, and banana has been shown by immunoblotting techniques (Lavaud et al. 1995). Moreover, two major banana allergens at 33 and 37 kDa have been identified, and they cross-react with latex (Delbourg et al. 1996).
Further cross-reactivity among latex, tomato and potato has been ascribed to a 46-kDa latex allergen, Hev b 7, sharing epitopes with a homologous protein (patatin) identified in potato (Beezhold et al. 1996). However, Hev b 7, patatins and their homologues appear not to contribute to cross-reactivity in the latex-fruit syndrome (Sowka et al. 1999). Although cross-reactivity of latex allergens with foods has been the objective of many investigations during the last few years (Lavaud et al. 1997, Nel & Gujuluva 1998), some of the cross-reacting allergens implicated in the latex-fruit syndrome have recently been isolated and well characterized. Table 3 summarizes the cross-reactions of latex allergens so far known.
Class I chitinases from chestnut and avocado have been purified, and a pool of sera from 4 latex-fruit allergic patients recognize these two proteins (Díaz-Perales et al. 1998). These class I chitinases include an N-terminal hevein-like domain in their sequence which could explain the latex-fruit cross-reactivity. The avocado class I chitinase has been cloned and expressed, and the cross-reactivity with hevein has been demonstrated (Sowka et al. 1998, Chen et al. 1998). Moreover, purified class I chitinases from chestnut and avocado elicited positive SPT in more than 50% of a group of 18 patients allergic to latex and fruits (Blanco et al. 1999). By contrast, SPT with class II chitinases from both foods, which lack the hevein-like domain, showed negative results. Furthermore, two major banana allergens have been characterized, and identified also as class I chitinases (Sanchez-Monge et al. 1999). The allergenicity of these banana allergens has been shown in more than 50% of a latex-banana allergic population (Sanchez-Monge et al. 1999), and cross-reactivity with hevein has also been demonstrated (Mikkola et al. 1998).
In another study, reactive proteins of 30 to 45 kDa (putative class I chitinases) were recognized by both specific policlonal antibodies to chitinases and sera from patients with latex-fruit allergy in chestnut, cherimoya, passion fruit, kiwi, papaya, mango, tomato, and wheat flour extracts (Díaz-Perales et al. 1999). Class I chitinase from avocado and a latex extract strongly inhibited IgE binding by these components when tested in immunoblot inhibition assays. These protein bands were not recognized by a pool of sera from patients allergic to latex but not to fruits. Joint consideration of the data discussed above indicates that these class I chitinases may be the panallergens responsible for the latex-fruit syndrome (Blanco et al. 1999).
Table 3: Latex allergens and their cross-reactivity (SB: spina bifida patients)
| Allergen | Identification | kDa | Allergenicity | Cross-reactivity or homologies |
| Hev b 1 | Rubber elongation factor (REF) | 14.6 / 58* | Major in SB | Papain |
| Hev b 2 | beta-1,3-Glucanase | 34-36 | Minor | Other glucanases |
| Hev b 3 | REF homologue | 24-27 | Major in SB | |
| Hev b 4 | Microhelix protein | 50-57 | Minor | |
| Hev b 5 | Acidic protein | 16 | Major | Kiwi acidic protein |
| Hev b 6 | Prohevein/hevein | 20/4.7 | Major | CBP 20 & PRP 4A (tobacco)
Win 1, Win 2 (Solanaceae) Class I chitinases |
| Hev b 7 | Patatin homologue | 43 | Minor | Patatin (Solanaceae) |
| Hev b 8 | Profilin | 14 | Minor | Panallergen |
| Hev b 9 | Enolase | 51 | Minor | |
| Hev b 10 | SO-dismutase | 26 | Minor | |
| - | Hevamine | 29 | Minor | Lysozyme |
THE DIAGNOSIS OF FOOD HYPERSENSITIVITIES ASSOCIATED WITH LATEX ALLERGY
In our experience, PPT with the fresh fruits implicated in the latex-fruit syndrome show an 80% concordance with the clinical diagnosis. PPT is an easy, cheap, and reproducible way to confirm the clinical suspicion of a fruit allergy. If you consider the fruits separately, the concordance is lower for the PPT with papaya and kiwi (around 60%, mainly due to some false positive results) than for banana, avocado, and chestnut, for which it is around 90% (Blanco 1997). In contrast, commercial extracts for skin prick tests (SPT) with the fruits implicated in the syndrome generally show a diagnostic sensitivity lower than 40%, with the exception of chestnut and kiwi commercial extracts, for which the concordance with the clinical history is around 80% (Blanco 1997). In agreement with our results, other authors have also found a more than 80% concordance between banana PPT and the clinical diagnosis (Delbourg at al. 1996).
In the same way, in our series of latex-food allergic patients, the
diagnostic sensitivity for fruit specific IgE was 37% (as determined by
the CAP System RAST FEIA, Pharmacia Diagnostics, Uppsala, Sweden), in relation
to the clinical history and PPT. Diagnostic sensitivity was better for
avocado specific IgE (around 80%) than for the rest of the fruits considered
(banana 50%, chestnut 25%, kiwi 20%). In our group of patients, diagnostic
specificity for fruit specific IgE determination was around 80%. In agreement
with our data, other authors have found a sensitivity of 32% for fruit
specific IgE, being better for avocado (67%) than for the rest of the foods
(Brehler et al. 1997). It was increased to 50% for patients reporting severe
reactions. Diagnostic specificity for fruit specific IgE determinations
was generally better than sensitivity, varying from 55% to 87% depending
on the food considered (Brehler et al. 1997).
PRACTICAL MANAGEMENT OF THE LATEX-FRUIT SYNDROME
Diagnosis of latex allergy is based on clinical history, complemented with SPT with a latex extract (Blanco et al. 1998, Hamilton & Adkinson 1998). In the same way, the diagnosis of the food hypersensitivities associated with latex allergy should be based on clinical history but complemented with PPT with fresh foods (Blanco et al. 1994). As stated previously, SPT with commercial food extracts shows a very variable diagnostic efficacy, while food specific IgE offers very poor predictive values, at least with respect to the fruits more commonly involved in the syndrome (Blanco et al. 1994, Brehler et al. 1997).
Figure 3 shows the algorithm used in our outpatient clinic for the diagnosis and management of the latex-fruit syndrome. Every patient allergic to latex, or to the foods more frequently involved in the syndrome, must be asked about possible adverse reactions to these foods. In these patients, PPT should be performed with banana, avocado, chestnut, and kiwi, as well as with the foods implicated in the reactions by each patient. In the case of severe anaphylaxis, a positive PPT is enough for the diagnosis. If the food adverse reaction was mild, or if there were several foods implicated, careful oral challenge tests should be considered.
In our opinion, foods commonly involved in the latex-fruit syndrome but not in adverse reactions for a given latex-allergic patient and which are not regularly consumed but elicit a clearly positive PPT should be avoided due to the risk of a severe reaction. In contrast, foods tolerated and regularly consumed should not be avoided, even if PPT is positive, although these patients should be advised of the risk. Foods showing negative PPT should not be avoided, and when in doubt, an oral challenge test is mandatory.
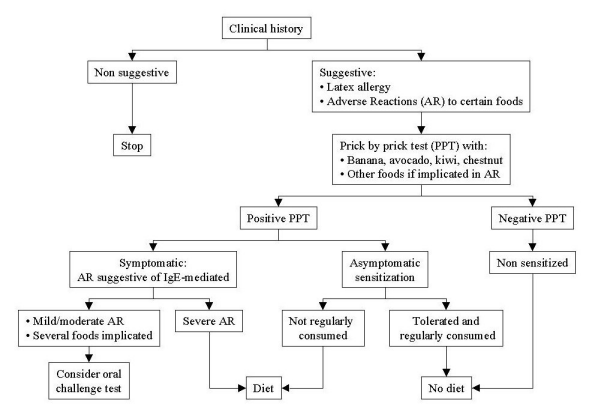 |
| Figure 3: Algorithm for the diagnose and management of the latex-fruit syndrome |
The latex-fruit syndrome is an example of cross reaction among allergens from distant species which has a clear clinical relevance. It should be kept in mind by all clinicians in order to prevent anaphylactic reactions to foods and/or latex. Recent data suggest that class I chitinases, with an N-terminal hevein-like domain, are new panallergens responsible for this syndrome. Future investigations should be focused on the development of better diagnostic tests and of new therapeutic approaches for this syndrome. In this context, recombinant panallergens might be useful both for diagnosis and for specific immunotherapy.