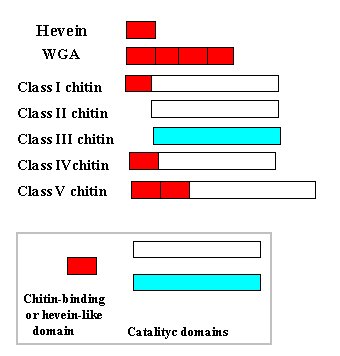
Figure 1: Structural scheme of proteins with hevein domains and chitinases
WGA: wheat germ agglutinin; Chitin: chitinases
The prevalence of latex allergy in the general population is low (less than 1%), but the prevalence of latex sensitization, measured as positive skin prick responses to natural rubber latex (NRL), or as IgE specific antibodies to latex proteins, is around 5% (Hadjiliadis et al.1995, Ownby et al.1996, Merret et al.1999). However, the latex allergy is considerably higher in health care workers (Watts et al.1998, Sinha & Harrison 1998, Vila et al.1999), patients who have had several surgical operations, such as those with spina bifida (Slater et al.1991, Cremer et al.1998, Shah et al.1998), and greenhouse workers (Carrillo et al.1995).
Around 50% of the patients with latex allergy show hypersensitivity to some plant foods, especially chestnut, banana and avocado, but also to kiwi, papaya, potato, tomato and others (Blanco et al.1994, Beezhold et al.1996, Brehler et al.1997, Lavaud et al.1997, Raulf-Heimsoth et al.1997). The term latex-fruit syndrome has been proposed to describe this type of cross-reactivity that probably involves allergens that share common epitopes in latex and these plant foods (Blanco et al.1994).
Up to 10 latex allergens have been identified and purified (see Breiteneder et al. 1998 for a recent review). Among them, hevein (Hev b 6.02) is a major allergen binding to IgE antibodies from more than 80% of the sera from latex allergic patients (Alenius et al.1995). It is a chitin binding, and probably a defence-related protein of 43 amino acid with antifungal properties. It is synthesized as a precursor of 20 kDa, prohevein, also present in the NRL. It has been demonstrated that the major IgE-binding epitopes of prohevein are in its N-terminal processed fragment hevein (Alenius et al.1996, Chen et al.1997, Beezhold et al.1997). The three-dimensional structure of hevein has been obtained by NMR spectroscopy (Asensio et al 1995) and several amino acids implicated in the binding to carbohydrates have been located (Asensio et al 1998).
There are some plant proteins, like wheat germ agglutinin, the potato win proteins, the antimicrobial peptides of amaranth, and some endochitinases, which have one or more domains homologous to hevein . Figure 1 shows the structure of wheat germ agglutinin, which has 4 hevein domains, along with that of the major classes of endochitinases based on Meins et al. (1994) and Neuhaus et al. (1996).
Endochitinases are enzymes widespread in nature. They catalyse the random cleavage of internal beta-1,4 glycosidic linkages in the chitin polymer. Chitin is a major component of fungal cell walls and of the exoskeleton of arthropods, organisms that include important plant pathogens and pests (see Collinge et al.1993 for a review) . A number of plant chitinases have been shown to inhibit fungal growth of various fungi in vitro (Schlumbaum et al.1986, Broekaert et al.1986, Mauch et al.1988). Moreover, the enhanced resistance to fungal pathogens in transgenic plants over-expressing chitinases (Broglie et al.1991, Jach et al.1995, Grisson et al.1996) strongly suggests a protective natural function of these proteins against plant pathogens. In fact, they were formally included in classes 3 and 4 of PR (pathogenesis related) plant proteins (See van Loon & van Strien 1999 for a recent review of the clasification and activities of PR proteins).
There are three chitinase classes, I, IV and V (see Fig.1) , with one or two chitin-binding domains more or less related to hevein. Class I, II and IV chitinases have homologous catalytic domains (with deletions in the latter case) and constitute family 19 of glycosyl hydrolases. Class III enzymes are included in family 18 of glycosyl hydrolases, which also comprises bacterial, fungal, and animal chitinases. A representative member of class V is the precursor of Urtica dioica agglutinin (Lerner & Raikhel 1992), which has a C-terminal domain similar to the catalytic domain of classes I and II chitinases and a N-terminal region with two chitin-binding domains. In natural rubber latex there are two minor allergens with chitinase activities: hevamine, a bifunctional lysozyme/chitinase enzyme homologous to class III chitinases (Jekel et al. 1991), and a class II enzyme (Posch et al. 1997).
The present paper reviews the results on class I chitinases containing a hevein-like domain as the principal panallergens implicated in the latex-fruit syndrome.
|
Figure 1: Structural scheme of proteins with hevein domains and chitinases WGA: wheat germ agglutinin; Chitin: chitinases |
 |
CLASS I CHITINASES ARE MAJOR ALLERGENS IMPLICATED IN THE CROSS-REACTIVITY OF CHESTNUT, AVOCADO AND BANANA WITH LATEX
Earlier studies pointed out that proteins of 30-35 kDa from chestnut, banana and avocado were the main IgE-binding proteins when sera from patients with latex-fruit syndrome were tested in vitro (Wadee et al.1990, Lavaud et al.1995, Delbourg et al.1996). Taking into account the molecular mass of class I chitinases (31 kDa), as well as the relationship between their N-terminal chitin-binding domain and latex hevein, such enzymes are likely candidates to be the major IgE-binding proteins in these fruits.
We have purified a class I and a class II (25 kDa) chitinase from chestnut and avocado and two isoforms of class I chitinases from banana (Diaz-Perales et al.1998, Sánchez-Monge et al. 1999, Blanco et al.1999). Three lines of evidence indicate that class I chitinases with a hevein-like domain, but not class II chitinases that lack this type of domain, are the main allergens of the latex-fruit syndrome.
i) In immunoblotting experiments, all the purified class I chitinases bound specific IgE antibodies from sera of latex-fruit allergic patients, and inhibited this binding on raw food extracts. Class II chitinases were not reactive.
ii) In RAST inhibition assays, class I enzymes reached inhibition values of 90-100%, whereas the value was considerably lower for class II chitinases.
iii) Skin prick tests (Table 1) were positive for all class I chitinases in more than 50% of the patients, but negative in all cases for the chestnut class II chitinase, and only positive in 2 out of 18 patients for the avocado class II enzyme.
Other groups have also identified class I chitinases as the major avocado (Sowka et al. 1998, Posch et al. 1999) and banana (Mikkola et al. 1998) allergens associated with the latex-fruit syndrome. Interestingly, purified latex hevein inhibits the specific IgE binding to these major allergens. Furthermore, hevein completely inhibited in RAST inhibition assays the anti-avocado IgE antibodies in 77% of latex allergic patients with avocado hypersensitivity (Chen et al. 1998, Posch et al. 1999).
Table 1: Skin prick test results of
patients with latex-fruit syndrome
| Sample* | % Positive SPT |
| Chestnut PBS extract | 89 |
| Purified chestnut class I chitinase Cas s 5 | 72 |
| Purified chestnut class II chitinase | 0 |
| Avocado PBS extract | 72 |
| Purified avocado class I chitinase Prs a 1 | 67 |
| Purified avocado class II chitinase | 11 |
| Banana PBS extract | 93 |
| Purified banana class I chitinase Mus a 1.1 | 53 |
| Purified banana class I chitinase Mus a 1.2 | 60 |
All these results suggest a central role for the hevein-like domain of class I chitinases in the hypersensitivity to chestnut, avocado and banana of latex allergic patients. As is shown in Figure 2, there is a sequence identity of about 70% between hevein and these domains in avocado, banana and chestnut that may explain this cross-reactivity. By contrast, the identity between hevein and chitin-binding domains of other proteins is only around 40%. The amino acid sequence of hevein includes the two IgE-epitopes found by Beezhold et al. (1997), which are shown in frames in Fig 2. The N-terminal epitope is highly conserved in wheat germ agglutinin (WgA) and could explain the cross-reactivity of this protein in some latex allergic patients. However, WgA neither binds to IgE of sera from latex-fruit syndrome patients nor inhibits the binding of class I chitinases (Diaz-Perales et al. 1998). This result suggests that different hevein-like domains show different immunological properties.
The data discussed above indicate that the main IgE-epitopes of class I chitinases must be located in their hevein-like domain. Our results also indicate that class I enzymes are recognised by sera from latex-fruit, but not from latex non-fruit allergic patients. Thus, it seems that the relevant IgE-epitopes are different for the two sera types (see also below).
 |
| Figure 2: Amino acid sequences of hevein-like domains and their
percentage of identity with latex hevein. Postulated IgE epitopes
of hevein are framed. Identities and differences of the other proteins
in comparison to hevein are indicated in blue and red, respectively:
Hev: Latex hevein, accesion number P02877; Prs a 1: avocado class I chitinase major allergen, Z78202; Cas s 5: chestnut class I chitinase major allergen, U48687; Mus a 1.1: banana class I chitinase major allergen, AF001524; Mus a 1.2: protein variant of the former; PvChI: green bean class I chitinase, P36361; WhChI: Wheat class I chitinase, Q41539; UcA-1: nettle agglutinin domain 1: P11218; WgA-1: wheat germ agglutinin domain 1, P10968; Win-1: potato wound-induced protein 1, X13497; WhChIV: Wheat class IV chitinase, AF112966; PvChIV: Green bean class IV chitinase, P27054; Ama-1: amaranth anti-microbial peptide 1, P27275. |
CLASS I ENDOCHITINASES AS PANALLERGENS OF THE LATEX-FRUIT SYNDROME
As was mentioned above, chitinases are widely distributed in plants and thus are present in several foods associated (fruits) or not associated (cereals and legumes) to the latex-fruit syndrome.
We have studied the presence of chitinases in 19 plant foods and their
role as cross-reactive determinants linked to latex-food allergy (Diaz-Perales
et al 1999). Protein extracts from fruits, nuts, vegetables, legumes and
cereals were immunodetected with a rabbit anti-chitinase serum, a pool
of sera from latex-fruit allergic patients, and a pool of sera from latex
allergic patients not showing fruit allergy . In six of the foods previously
associated with the syndrome (cherimoya, passion fruit, kiwi, papaya, mango
and tomato), both monospecific polyclonal anti-chitinase antibodies and
sera from patients with latex-fruit allergy recognised the same proteins
with approximately 30 to 45 kDa. In wheat, not associated to the latex-fruit
syndrome, there are also IgE binding chitinases. In immunoblot inhibition
assays Prs a 1, the class I chitinase, and major avocado allergen, as well
as a crude latex extract, strongly or fully inhibited the IgE binding of
these proteins. Thus, besides in chestnut, avocado and banana, putative
class I chitinases also seem to be the cross-reactive components in other
fruits associated with the latex-fruit-syndrome. Moreover, these allergens
are not recognised by sera from latex allergic individuals without fruit
allergy.
CLASS I CHITINASES ARE ETHYLENE INDUCIBLE AND HEAT INACTIVATED
The presence of IgE-binding chitinases in plant foods not associated with the latex-fruit syndrome is an apparent contradiction. This fact has been further investigated using Phaseolus vulgaris as a model system (Sánchez-Monge et al. 2000). Green bean is an extensively consumed legume that has not been implicated in the syndrome. It has also been proved that this specie has and expresses genes encoding class I chitinases (Broglie et al.1986).
A 32 kDa green bean protein recognised by both monospecific polyclonal antibodies against plant chitinases and sera from latex-fruit allergic patients has been isolated (Sánchez-Monge et al. 2000). Its allergenicity was confirmed by positive skin prick test responses in 7 out of 8 patients suffering the latex-fruit syndrome. N-terminal amino acid sequencing allowed us to identify the protein as a bean class I chitinase highly related (» 75% identity) to the major avocado allergen Prs a 1. Cross-reactivity between both proteins was demonstrated by immunoblot inhibition assays. Thus, the presence in raw green beans of an allergen homologous to those described in avocado, banana and chestnut was demonstrated.
The amount of the bean allergen greatly increased in response to ethylene treatment.
Class I chitinases from other foods, such as banana (Clendennen & May 1997, Medina-Suarez et al.1997), show similar induction. Ethylene is a plant hormone commercially used to hasten fruit ripening of several climacteric fruits, including apple, avocado, banana and tomato. These observations might be relevant, as they indicate that the levels of these allergens can strongly vary during fruit ripening and with ethylene treatment.This fact could explain, at least in part, the rise in the prevalence of the latex-fruit allergy which has been observed in recent years.
The characterisation of the green bean allergen has uncovered the potential presence of cross-reactive class I chitinases in a wide array of plant foods. However, it seems that only those that are consumed raw, like fruits, are strongly associated with latex allergy. In contrast, foods consumed after heat treatment ( boiling or cooking) do not usually cross-react with latex. We have shown (Sánchez-Monge et al. 2000) that both avocado (Prs a 1) and bean class I chitinases lose their in vitro IgE-binding capacity after boiling. Moreover, not positive skin prick test responses were obtained when the heat-treated allergens were proved in latex-fruit allergic patients who clearly reacted to the untreated allergens. The inactivation has also been observed in commercial canned and frozen green beans, which are usually heat treated during their industrial processing, as well as in chestnut commercial derivatives like puree and syrup. It seems that heat treatment inactivates the allergenic capacity of class I chitinase allergens, and thus only foods consumed raw, but not those which undergo industrial or home heating treatment, are associated with the latex-fruit syndrome.
The evidence accumulated so far suggests that class I chitinases are the major panallergens associated with the latex-fruit syndrome. cDNA clones for some of these allergenic enzymes have been obtained (Clendennen et al.1997, Allona et al.1996), and the corresponding recombinant proteins have been expressed in yeast (Sowka et al. 1998, Diaz-Perales et al. unpublished). These recombinant products may be useful for the diagnostic and potential immunotherapy of latex related allergy. On the other hand, protein engineering should help to locate the major IgE-epitopes in these allergens and provide hypoallergenic variants. Particular attention should be paid to their hevein-like domains. Their inactivation by heating and their induction by hormone treatment can be relevant from a clinical point of view.
Acknowledgements
We thank Dr. Luis Gomez for carefully reading of the manuscript. This work was supported by Dirección General de Enseñanza Superior e Investigación Cientifíca, MEC (grant PB98-0735).