Hypersensitivity to hazelnut and the cross reactivity between hazelnut proteins and different tree pollen proteins, such as birch, hazel, alder, hornbeam, oak, or mugwort, are well known (Halmepuro et al. 1984, Calkoven et al. 1987, Dreborg 1988, Hirschwehr et al. 1992, Caballero et al. 1994, Asero 1997, Caballero et al. 1997). Although hazelnuts are considered some of the most common causes of food allergies among both children (Crespo et al. 1995) and adults (André et al. 1994) in Europe, there have been only a few investigations regarding the responsible allergenic proteins in hazelnuts (Hirschwehr et al. 1992, Caballero et al. 1997). By means of SDS-PAGE and immunoblotting an 18 kDa protein as the major allergen in hazelnuts and an additional IgE-binding profilin at 14 kDa have been detected , which are related to the hazel pollen allergens Cor a 1 (17 kDa) and profilin (14 kDa) (Hirschwehr et al. 1992). Recently the stability of hazelnut allergens against heat treatment has been demonstrated, but only a little is known about the influence of processing or enzymatic treatment on the allergenicity of the hazelnut allergens (Wigotzki et al. 1999).
The aim of the present study was to investigate the influence of enzymatic treatment with typical proteolytic enzymes, such as pepsin, pancreatin, trypsin, elastase and protease on the allergenicity of the main allergens in hazelnuts by means of SDS-PAGE, immunoblotting and enzyme allergosorbent test (EAST) inhibition.
Human Sera
A total of 15 patients' sera were collected at the University
Hospital Eppendorf, Department of Dermatology and Allergology, Hamburg,
Germany. Serum Mast 127 was supplied by Mast Diagnostica, Reinfeld, Germany.
The relevant clinical information is summarized in Table 1. Anamnesis of
the patients reveals further sensitivities to different fruits, nuts and
tree pollen. All sera were EAST class >/= 2 when tested for specific IgE
against hazelnut protein extract by enzyme allergosorbent test (Allergopharma,
Reinbek, Germany). Pool-serum was collected using equal aliquots of 13
sera with specific IgE-binding against hazelnut (sera 1-13). A serum from
a non-atopic individual with no history of food hypersensitivity (EAST
class 0) was used as negative control.
Table 1: Characterization of patients with hazelnut sensitivity
| No | Patient
(Sex, age) |
Clinical Symptoms |
EAST class |
Further Sensitivities (Anamnesis) |
|
|
TR (male, 34) | IM, SL, DB, HB |
|
apple, cherry, plum, peanut |
|
|
TS (male, 27) | IT |
|
hens egg, wheat, milk, yeast, pineapple, apple, birch pollen, grass pollen |
|
|
LS (male, 27) | IT |
|
apple, birch pollen |
|
|
SK (female, 28) | IM |
|
peach, apple, pear, strawberry, birch pollen, hazel pollen |
|
|
UW (male, 26) | IM, SL, DB |
|
apple, potato, walnut, birch pollen, hazel pollen, alder pollen |
|
|
BS 127 (nk, nk) | nk |
|
nk |
|
|
IB (female, 29) | IM, IT |
|
celery, carrot, mango, mugwort pollen, birch pollen |
|
|
HN (male, 25) | IM, SL |
|
walnut |
|
|
IK (female, 28) | IM, SL |
|
nk |
|
|
SM (male, 29) | IM, IT |
|
almond, birch pollen |
|
|
BvW (female, 40) | IM, IT |
|
apple, cherry, plum |
|
|
HRF (female, 52) | IM, IT |
|
birch pollen, alder pollen, hazel pollen |
|
|
JA (female, 26) | IM, SL |
|
almond, apple, tree pollen, grass pollen |
|
|
MM (female, 20) | IM, SL, IT, DB, N |
|
apple, potato, birch pollen |
|
|
TV (male, 22) | IM, N |
|
apple, birch pollen |
|
|
KDH (male, 45) | IM |
|
carrot, potato, apple, mango, litchi, birch pollen, mugwort pollen |
Hazelnuts and Enzymes
The hazelnuts used in our investigations were purchased
at a local food store. Protease (from Tritirachium album, 13.4 U/mg), pancreatin
(from porcine pancreas, 4 x USP) and trypsin (from bovine pancreas, 10
U/mg) were purchased from Sigma Chemical Co. (St. Louis, MO), pepsin (from
porcine stomach, 0.7 FIP-U/mg) was purchased from Merck (Darmstadt, Germany)
and elastase (from porcine pancreas, 3750 U/mg) was purchased from ICN
(Aurora, Ohio).
Native Allergen Extracts
Native protein extracts were obtained by extracting 1
g of the raw peeled and ground hazelnuts with 30 mL phosphate buffered
saline [PBS (0.01 M potassium phosphate buffer, pH 7.4, 0.13 M sodium
chloride)] for 60 min at room temperature. The suspension was centrifuged
(4 °C, 10500 g, 60 min) and the supernatant filtered. The solution
was divided into aliquots of 2 ml, lyophilized and stored at -20 °C
until use.
In Vitro Digestion
Digestion was carried out with peeled and ground hazelnuts
according to Asselin et al. (1989). 10 g of the nuts were mixed with
50 mg of each enzyme and 50 mL PBS. pH was adjusted in the case of pepsin
to 1.2 with HCl and in the case of elastase to 8.8 with NaOH. The solutions
were incubated at 37°C for 30, 60 and 240 min. The enzyme reaction
was terminated by heating to 90°C for 20 min. Decrease of IgE- binding
activity by heating was not expected, scince hazelnut allergens were shown
to be heat stable for at least 90 min at 100°C (dry heating) in EAST-
inhibition and immunoblotting experiments (Wigotzki et al. 1999). The digested
nuts were centrifuged and filtered. The filtered extracts were used for
further investigations.
Protein Determination
The protein contents of the extracts were determined
by the method of Bradford (1976).
SDS-PAGE
SDS-PAGE was performed in both tricine gels (80 mm x
80 mm x 1.5 mm) containing 16 % acrylamide and 10 % acrylamide gels using
NuPAGE® vertical electrophoresis system according to the manufacturer's
recommendations (Novex, San Diego, CA, USA). Protein samples dissolved
in Tris-HCl/SDS sample buffer (pH 6.8) containing 5 % (w/v) beta- mercaptoethanol
(Vieths et al. 1992) were boiled for 3 min. Applying about 20 µg
protein per well, electrophoresis was performed at 200 V constant voltage
for 1.5 h on the NuPAGE® electrophoresis system (Novex, San Diego,
CA, USA) using a MultiDrive XL power supply (Pharmacia LKB, Uppsala, Sweden).
Gels were silver stained according to Heuckeshoven and Dernick (1990).
Immunoblotting
Proteins were electrotransferred from slab gels to nitrocellulose
(NC membrane 0.2 µm, Schleicher & Schuell, Dassel, Germany) at
0.8 mA/cm2 for 80
min using a NovaBlot semidry blotting apparatus (Pharmacia LKB, Uppsala,
Sweden) according to Kyhse-Andersen (1984) with discontinuous buffer system
as described by Vieths et al. (1992). Afterwards the membrane was dried
for 30 min and cut into strips of 2 mm. Immunostaining of IgE was performed
by the method of Vieths et al. (1992), slightly modified as described previously
by Möller et al. (1997). To check the efficiency of protein transfer,
from each gel one strip including separated proteins and a molecular mass
(Mr) marker (Pharmacia LKB, Uppsala, Sweden) was stained with colloidal
gold staining (Bio-Rad, Munich, Germany) according to Moeremans et al.
(1985).
Enzyme Allergosorbent Test (EAST) Inhibition
For inhibition experiments the pooled serum was diluted
1+1 in incubation buffer (PBS containing 0.3 % (w/v) bovine serum albumin
(BSA) and 0.1 % (v/v) Tween 20). A 10-fold dilution series of the inhibitor
extracts from native and digested hazelnuts was prepared in five to six
steps using the same incubation buffer. Nonspecific inhibition was checked
with ovalbumin. Possible inhibitions caused by the enzyme preparations
were checked as well. 50 µL of the diluted serum were mixed with
50 µL of the inhibitor solution and an allergen disk. The solutions
were incubated overnight at room temperature in the dark. After washing
three times with 1 % Tween 20 in PBS (v/v), 50 µL of anti-human IgE
alkaline phosphatase conjugate (Allergopharma, Reinbek, Germany), diluted
1+1 in incubation buffer, were added and incubated overnight. The disks
were washed again and enzyme activity was stained with p-nitrophenylphosphate
(PNPP) for 60 min at 37°C. Absorbance was measured at 405 nm (Möller
et al., 1997).
SDS-PAGE
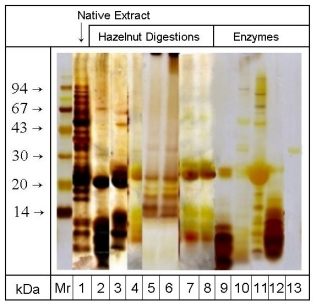
Figure 1 illustrates the silver stained enzyme preparations and protein
extracts of the native and digested hazelnuts separated by SDS-PAGE using
a 10% acrylamide gel. The Mr marker protein bands ranged from 14 kDa to
94 kDa. Each of the different enzymatic treatments led to a strong degradation
of proteins > 20 kDa. In the case of digestion with elastase, protease
and trypsin (lanes 2-4) additional proteins with molecular masses at 16
kDa and 18 kDa were degraded. After peptic digestion (lanes 5 and 6) protein
bands between 20 kDa and 14 kDa were detectable, whilst proteins with molecular
masses > 20 kDa and < 14 kDa were degraded. Pancreatic digestion led
to strong degradation of proteins > 20 kDa and between 20 and 14 kDa (lanes
7 and 8). Since each of the enzyme preparations except for pepsin reveals
protein bands at 20 kDa and in the region < 14 kDa (lanes 9-13), the
distinction between enzyme preparation and hazelnut proteins in these molecular
mass regions is not possible.
| Figure 1: SDS-PAGE/silver staining of proteins from native and digested hazelnut extracts and enzyme preparations. Mr: molecular mass marker proteins, 1: native hazelnut extract, 2: extract after 30 min protease digestion, 3: extract after 30 min trypsin digestion, 4: extract after 30 min elastase digestion, 5: extract after 60 min pepsin digestion, 6: extract after 240 min pepsin digestion, 7: extract after 30 min pancreatin digestion, 8: extract after 60 min pancreatin digestion, 9: trypsin preparation, 10: pancreatin preparation, 11: elastase preparation, 12: protease preparation, 13: pepsin preparation. (Gel: 10% acrylamide) |  |
Immunoblotting
The protein extracts of the different digested hazelnuts were characterized
by SDS-PAGE / immunoblotting using seven sera from hazelnut allergic patients.
Table 2 reveals the allergens detected in the native hazelnut extract by
the sera used for immunoblotting experiments (the immunoblot of the native
extract is identical to the one after 60 min pepsin treatment shown in
Figure 2). Each of the sera detected the 18 kDa major hazelnut allergen.
Sera 1, 5 and 6 detected an additional 14 kDa protein. Sera 3, 6 and 7
revealed a further allergen band of approximately 30 kDa, whilst serum
2 showed a wide range of IgE binding proteins between 30 kDa and 70 kDa.
These results are comparable to the results of Hirschwehr et al. (1992),
who detected an 18 kDa protein and an additional 14 kDa protein as major
allergens in hazelnuts and further proteins at 37 kDa, 40 kDa, 46 kDa and
69 kDa. A 20 kDa protein, which was strongly detected by means of silver
staining, showed no IgE binding activity in immunoblotting experiments.
Table 2: Allergens in native hazelnut extract. Detection with 7 patients'
sera in SDS-PAGE / immunoblot
|
|
|
|
|
|
|
|
(Hirschwehr et al. 1992) |
|
14 kDa |
18 kDa |
30 kDa 18 kDa |
|
14 kDa |
18 kDa 14 kDa |
18 kDa |
46 kDa 40 kDa 37 kDa 18 kDa 14 kDa |
After 30 min digestion with protease, elastase and trypsin IgE-binding activities were no longer detectable (results not shown). Peptic digestion resulted in a distinct detection of the allergens after 60 min treatment and even in a weak detection by two of the sera (1 and 2) after 240 min (Fig 2). The results of immunoblotting after 60 min treatment did not differ from the results using the native extract (not shown).
In the case of pancreatin 60 min digestion was necessary
to eliminate the allergenic activity of the hazelnut protein extract completely.
30 min treatment led to an elimination of the allergen bands of approximately
30 kDa by detection with sera 3, 6 and 7, whilst each of the seven sera
still detected the 18 kDa major allergen (Fig 3). The 14 kDa allergen was
detected distinctly by serum 1 and weakly by sera 4 and 6.
| Figure 2: SDS-PAGE/Immunoblotting of hazelnut extracts treated with pepsin. Mr: colloidal gold stained molecular mass marker proteins, 1-7: patients' sera (MM, KDH, TR, UW, JA, TV, and LS as described in Table 1), 8: control serum, left side: 60 min treatment, right side: 240 min treatment. (Gel: 16% acrylamide) | 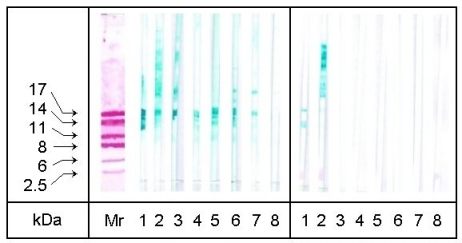 |
| Figure 3: SDS-PAGE/Immunoblotting of hazelnut extracts treated with pancreatin. Mr: colloidal gold stained molecular mass marker proteins, 1-7: patients' sera (MM, KDH, TR, UW, JA, TV, and LS as described in Table 1), 8: control serum, left side: 30 min treatment, right side: 60 min treatment. (Gel: 16% acrylamide) | 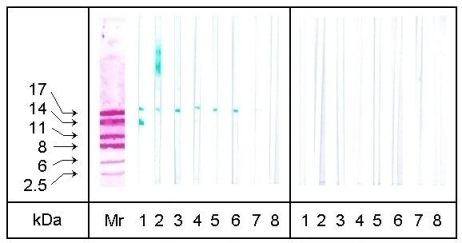 |
EAST Inhibition
The results of the EAST inhibition investigations of
the allergen extracts of the digested hazelnuts using the pooled serum
of 13 allergic individuals are illustrated in figures 4-6. The resulting
C50-concentrations of the digested hazelnut proteins responsible for a
50% inhibition of the IgE binding to the solid phase are listed in the
legends.
Figure 4 reveals the inhibition graphs of the hazelnuts
digested 30 min with protease, elastase and trypsin. In addition the graph
of a native undigested hazelnut extract is shown. Whilst the native extract
shows a typical inhibition curve with a C50-concentration of 38 mg/L, none
of the three digested extracts shows inhibition values > 30%, which means
a weak allergenic activity of each extract. Controls with ovalbumin and
the enzyme preparations did not result in any inhibition.
| Figure 4: EAST-inhibition of IgE-binding to hazelnut proteins by 30 min enzymatically treated hazelnut extracts. Inhibitors: native hazelnut extract (C50-concentration = 38 mg/L), trypsin treated extract, protease treated extract, elastase treated extract. | 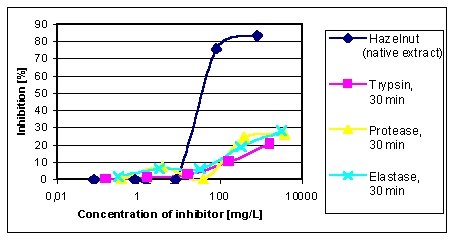 |
The inhibition graphs in figure 5 were obtained by the native hazelnut
extract and the extracts after digestion with pepsin at 60 min and 240
min. The C50-concentrations slightly decrease from native extract (35 mg/L)
to the extract after 60 min digestion (128 mg/L), whilst the extract after
240 min digestion shows a maximum inhibition of 34%, which means a distinct
decrease of allergenic activity. Controls with ovalbumin and the pepsin
preparation did not result in any inhibition.
| Figure 5: EAST-inhibition of IgE-binding to hazelnut proteins by peptic treated hazelnut extracts. Inhibitors: native hazelnut extract (C50-concentration = 35 mg/L), 60 min treated extract (C50-concentration = 128 mg/L), 240 min treated extract. | 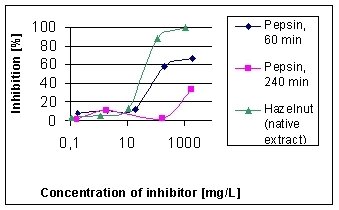 |
Figure 6 reveals the EAST inhibition results of native hazelnut extract
and the extracts treated 30 and 60 min with pancreatin. Whilst 30 min treatment
leads to a C50-concentration of 143 mg/L (native extract: 38 mg/L), 60
min treatment results in a maximum inhibition of 31%, which means a distinct
decrease of allergenic activity. Controls with ovalbumin and the pancreatin
preparation did not result in any inhibition.
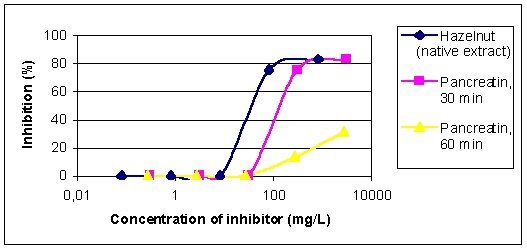 |
| Figure 6: EAST-inhibition of IgE-binding to hazelnut proteins by pancreatic treated hazelnut extracts. Inhibitors: native hazelnut extract (C50-concentration = 38 mg/L), 30 min treated extract (C50-concentration = 143 mg/L), 60 min treated extract. |
Although hazelnuts are an important cause of food allergies in Europe and many reports have been published concerning the sensitization to hazelnuts and the characterization of the allergens (Hirschwehr et al. 1992, Caballero et al. 1997), there are only a few studies about their stability (Wigotzki et al. 1999). The effect of proteolytic digestion is an important factor with regard to the allergenicity of food proteins and may be an important condition for food allergens to initiate a sensitization via the gastrointestinal tract.
In our study we incubated the ground hazelnuts with different enzyme preparations. In the case of protease, elastase and trypsin the allergenic activity already decreased after 30 min treatment. Whilst EAST-inhibition experiments revealed residual allergenic activities, no allergen bands were detectable by immunoblotting. A possible explanation for the higher sensitivity of EAST-inhibition compared to immunoblot results could be the formation of low molecular mass peptides, which are no longer detectable by electrophoretic methods.
Although the stability to peptic and pancreatic digestion was higher than digestion with protease, elastase and trypsin, in summary the hazelnut allergens can be described as instable to enzymatic digestion, particularly because the applied conditions of digestion according to Asselin et al. (1989) are based on an enzyme content of 0.1 %, which is lower than described for standard simulated gastric and intestinal fluids in The United States Pharmacopeia (1995). After a complete digestion consisting of peptic followed by pancreatic treatment the elimination of IgE binding activities of the hazelnut proteins can be expected.
Our results agree with investigations by Burks et al. (1991), who found a significant reduction in IgE-binding of a digested soy product, but they are in contrast to a study by Dominguez et al. (1990), who showed a high resistance of a mustard allergen to degradation by proteolytic enzymes. A further study Astwood et al. (1996) revealed the stability of various important food allergens to digestion in simulated gastric fluid. Unlike our investigations and the investigations by Burks et al. (1991) those studies were done with the purified allergen extracts. Matrix influences were not taken into consideration, although this is an important prerequisite determining the allergenicity of food proteins. In a further work Vieths et al. (1999) investigated food extracts of peanuts and hazelnuts after 2 h of gastric digestion followed by 45 min treatment under duodenal conditions. In the case of hazelnuts the IgE reactivity of the untreated sample was reduced to < 10% by both gastric and combined gastric-duodenal digestion for a pooled serum from 4 patients. These results are comparable to our results.